Sclerostin inhibition reverses skeletal fragility in an Lrp5-deficient mouse model of OPPG syndrome
- PMID: 24225945
- PMCID: PMC3964772
- DOI: 10.1126/scitranslmed.3006627
Sclerostin inhibition reverses skeletal fragility in an Lrp5-deficient mouse model of OPPG syndrome
Abstract
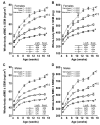
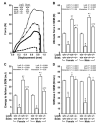
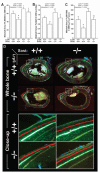
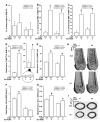
Osteoporosis pseudoglioma syndrome (OPPG) is a rare genetic disease that produces debilitating effects in the skeleton. OPPG is caused by mutations in LRP5, a WNT co-receptor that mediates osteoblast activity. WNT signaling through LRP5, and also through the closely related receptor LRP6, is inhibited by the protein sclerostin (SOST). It is unclear whether OPPG patients might benefit from the anabolic action of sclerostin neutralization therapy (an approach currently being pursued in clinical trials for postmenopausal osteoporosis) in light of their LRP5 deficiency and consequent osteoblast impairment. To assess whether loss of sclerostin is anabolic in OPPG, we measured bone properties in a mouse model of OPPG (Lrp5(-/-)), a mouse model of sclerosteosis (Sost(-/-)), and in mice with both genes knocked out (Lrp5(-/-);Sost(-/-)). Lrp5(-/-);Sost(-/-) mice have larger, denser, and stronger bones than do Lrp5(-/-) mice, indicating that SOST deficiency can improve bone properties via pathways that do not require LRP5. Next, we determined whether the anabolic effects of sclerostin depletion in Lrp5(-/-) mice are retained in adult mice by treating 17-week-old Lrp5(-/-) mice with a sclerostin antibody for 3 weeks. Lrp5(+/+) and Lrp5(-/-) mice each exhibited osteoanabolic responses to antibody therapy, as indicated by increased bone mineral density, content, and formation rates. Collectively, our data show that inhibiting sclerostin can improve bone mass whether LRP5 is present or not. In the absence of LRP5, the anabolic effects of SOST depletion can occur via other receptors (such as LRP4/6). Regardless of the mechanism, our results suggest that humans with OPPG might benefit from sclerostin neutralization therapies.
Figures

Comment in
-
Insights into the mechanisms of sclerostin action in regulating bone mass accrual.J Bone Miner Res. 2014 Jan;29(1):24-8. doi: 10.1002/jbmr.2154. J Bone Miner Res. 2014. PMID: 24285419 No abstract available.
-
Bone diseases: Sclerostin neutralization--a viable pathway for OPPG?Nat Rev Rheumatol. 2014 Jan;10(1):4. doi: 10.1038/nrrheum.2013.189. Epub 2013 Dec 3. Nat Rev Rheumatol. 2014. PMID: 24296680 No abstract available.
References
-
- Becker DJ, Kilgore ML, Morrisey MA. The societal burden of osteoporosis. Curr. Rheumatol. Rep. 2010;12:186–191. - PubMed
-
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner. Res. 2007;22:465–475. - PubMed
-
- Robbins J, Aragaki AK, Kooperberg C, Watts N, Wactawski-Wende J, Jackson RD, LeBoff MS, Lewis CE, Chen Z, Stefanick ML, Cauley J. Factors associated with 5-year risk of hip fracture in postmenopausal women. JAMA. 2007;298:2389–2398. - PubMed
-
- Gong Y, B. Slee R, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Jüppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. Osteoporosis-Pseudoglioma Syndrome Collaborative Group, LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. - PubMed
-
- Saarinen A, Saukkonen T, Kivelä T, Lahtinen U, Laine C, Somer M, Toiviainen-Salo S, Cole WG, Lehesjoki AE, Mäkitie O. Low density lipoprotein receptor-related protein 5 (LRP5) mutations and osteoporosis, impaired glucose metabolism and hypercholesterolaemia. Clin. Endocrinol. 2010;72:481–488. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

