Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia
- PMID: 24226519
- PMCID: PMC3920019
- DOI: 10.1152/ajprenal.00518.2013
Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia
Abstract
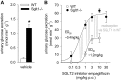
In the kidney, the sodium-glucose cotransporters SGLT2 and SGLT1 are thought to account for >90 and ∼3% of fractional glucose reabsorption (FGR), respectively. However, euglycemic humans treated with an SGLT2 inhibitor maintain an FGR of 40-50%, mimicking values in Sglt2 knockout mice. Here, we show that oral gavage with a selective SGLT2 inhibitor (SGLT2-I) dose dependently increased urinary glucose excretion (UGE) in wild-type (WT) mice. The dose-response curve was shifted leftward and the maximum response doubled in Sglt1 knockout (Sglt1-/-) mice. Treatment in diet with the SGLT2-I for 3 wk maintained 1.5- to 2-fold higher urine glucose/creatinine ratios in Sglt1-/- vs. WT mice, associated with a temporarily greater reduction in blood glucose in Sglt1-/- vs. WT after 24 h (-33 vs. -11%). Subsequent inulin clearance studies under anesthesia revealed free plasma concentrations of the SGLT2-I (corresponding to early proximal concentration) close to the reported IC50 for SGLT2 in mice, which were associated with FGR of 64 ± 2% in WT and 17 ± 2% in Sglt1-/-. Additional intraperitoneal application of the SGLT2-I (maximum effective dose in metabolic cages) increased free plasma concentrations ∼10-fold and reduced FGR to 44 ± 3% in WT and to -1 ± 3% in Sglt1-/-. The absence of renal glucose reabsorption was confirmed in male and female Sglt1/Sglt2 double knockout mice. In conclusion, SGLT2 and SGLT1 account for renal glucose reabsorption in euglycemia, with 97 and 3% being reabsorbed by SGLT2 and SGLT1, respectively. When SGLT2 is fully inhibited by SGLT2-I, the increase in SGLT1-mediated glucose reabsorption explains why only 50-60% of filtered glucose is excreted.
Keywords: diabetes mellitus; glucose reabsorption; glucose transport; proximal tubule; sodium glucose cotransport inhibitor.
Figures



References
-
- Balen D, Ljubojevic M, Breljak D, Brzica H, Zlender V, Koepsell H, Sabolic I. Revised immunolocalization of the Na+-d-glucose cotransporter SGLT1 in rat organs with an improved antibody. Am J Physiol Cell Physiol 295: C475–C489, 2008 - PubMed
-
- DeFronzo RA, Davidson JA, del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab 14: 5–14, 2012 - PubMed
-
- Gorboulev V, Schurmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R, Veyhl-Wichmann M, Srinivasan A, Balen D, Breljak D, Rexhepaj R, Parker HE, Gribble FM, Reimann F, Lang F, Wiese S, Sabolic I, Sendtner M, Koepsell H. Na+-d-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion Diabetes 61: 187–196, 2012 - PMC - PubMed
-
- Grempler R, Thomas L, Eckhardt M, Himmelsbach F, Sauer A, Sharp DE, Bakker RA, Mark M, Klein T, Eickelmann P. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab 14: 83–90, 2012 - PubMed
-
- Heise T, Seewaldt-Becker E, Macha S, Hantel S, Pinnetti S, Seman L, Woerle HJ. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks' treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab 15: 613–621, 2013 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

