N-glycosylation requirements in neuromuscular synaptogenesis
- PMID: 24227656
- PMCID: PMC3848190
- DOI: 10.1242/dev.099192
N-glycosylation requirements in neuromuscular synaptogenesis
Abstract
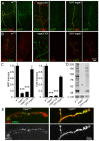
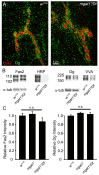
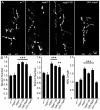
Neural development requires N-glycosylation regulation of intercellular signaling, but the requirements in synaptogenesis have not been well tested. All complex and hybrid N-glycosylation requires MGAT1 (UDP-GlcNAc:α-3-D-mannoside-β1,2-N-acetylglucosaminyl-transferase I) function, and Mgat1 nulls are the most compromised N-glycosylation condition that survive long enough to permit synaptogenesis studies. At the Drosophila neuromuscular junction (NMJ), Mgat1 mutants display selective loss of lectin-defined carbohydrates in the extracellular synaptomatrix, and an accompanying accumulation of the secreted endogenous Mind the gap (MTG) lectin, a key synaptogenesis regulator. Null Mgat1 mutants exhibit strongly overelaborated synaptic structural development, consistent with inhibitory roles for complex/hybrid N-glycans in morphological synaptogenesis, and strengthened functional synapse differentiation, consistent with synaptogenic MTG functions. Synapse molecular composition is surprisingly selectively altered, with decreases in presynaptic active zone Bruchpilot (BRP) and postsynaptic Glutamate receptor subtype B (GLURIIB), but no detectable change in a wide range of other synaptic components. Synaptogenesis is driven by bidirectional trans-synaptic signals that traverse the glycan-rich synaptomatrix, and Mgat1 mutation disrupts both anterograde and retrograde signals, consistent with MTG regulation of trans-synaptic signaling. Downstream of intercellular signaling, pre- and postsynaptic scaffolds are recruited to drive synaptogenesis, and Mgat1 mutants exhibit loss of both classic Discs large 1 (DLG1) and newly defined Lethal (2) giant larvae [L(2)GL] scaffolds. We conclude that MGAT1-dependent N-glycosylation shapes the synaptomatrix carbohydrate environment and endogenous lectin localization within this domain, to modulate retention of trans-synaptic signaling ligands driving synaptic scaffold recruitment during synaptogenesis.
Keywords: Active zone; Drosophila; Glutamate receptor; Neuromuscular junction; Synaptic scaffold; Synaptomatrix; Trans-synaptic signaling.
Figures





References
-
- Abramoff M. D., Magalhaes P. J., Ram S. J. (2004). Image processing with ImageJ. Biophotonics International 11, 36–42
-
- Beumer K. J., Rohrbough J., Prokop A., Broadie K. (1999). A role for PS integrins in morphological growth and synaptic function at the postembryonic neuromuscular junction of Drosophila. Development 126, 5833–5846 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

