Tau pathology is present in vivo and develops in vitro in sensory neurons from human P301S tau transgenic mice: a system for screening drugs against tauopathies
- PMID: 24227726
- PMCID: PMC3828468
- DOI: 10.1523/JNEUROSCI.4933-12.2013
Tau pathology is present in vivo and develops in vitro in sensory neurons from human P301S tau transgenic mice: a system for screening drugs against tauopathies
Abstract
Intracellular tau aggregates are the neuropathological hallmark of several neurodegenerative diseases, including Alzheimer's disease, progressive supranuclear palsy, and cases of frontotemporal dementia, but the link between these aggregates and neurodegeneration remains unclear. Neuronal models recapitulating the main features of tau pathology are necessary to investigate the molecular mechanisms of tau malfunction, but current models show little and inconsistent spontaneous tau aggregation. We show that dorsal root ganglion (DRG) neurons in transgenic mice expressing human P301S tau (P301S-htau) develop tau pathology similar to that found in brain and spinal cord and a significant reduction in mechanosensation occurs before detectable fibrillar tau formation. DRG neuronal cultures established from adult P301S-htau mice at different ages retained the pattern of aberrant tau found in vivo. Moreover, htau became progressively hyperphosphorylated over 2 months in vitro beginning with nonsymptomatic neurons, while hyperphosphorylated P301S-htau-positive neurons from 5-month-old mice cultured for 2 months died preferentially. P301S-htau-positive neurons grew aberrant axons, including spheroids, typically found in human tauopathies. Neurons cultured at advanced stages of tau pathology showed a 60% decrease in the fraction of moving mitochondria. SEG28019, a novel O-GlcNAcase inhibitor, reduced steady-state pSer396/pSer404 phosphorylation over 7 weeks in a significant proportion of DRG neurons showing for the first time the possible beneficial effect of prolonged dosing of O-GlcNAcase inhibitor in vitro. Our system is unique in that fibrillar tau forms without external manipulation and provides an important new tool for understanding the mechanisms of tau dysfunction and for screening of compounds for treatment of tauopathies.
Figures



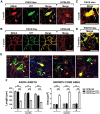



References
-
- Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, Atzori C, Migheli A, Crowther RA, Ghetti B, Spillantini MG, Goedert M. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci. 2002;22:9340–9351. - PMC - PubMed
-
- Biernat J, Mandelkow EM, Schröter C, Lichtenberg-Kraag B, Steiner B, Berling B, Meyer H, Mercken M, Vandermeeren A, Goedert M, et al. The switch of tau protein to an Alzheimer-like state includes the phosphorylation of two serine-proline motifs upstream of the microtubule binding region. EMBO J. 1992;11:1593–1597. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
