Uncoupling of EphA/ephrinA signaling and spontaneous activity in neural circuit wiring
- PMID: 24227729
- PMCID: PMC3831575
- DOI: 10.1523/JNEUROSCI.1931-13.2013
Uncoupling of EphA/ephrinA signaling and spontaneous activity in neural circuit wiring
Abstract
Classic studies have proposed that genetically encoded programs and spontaneous activity play complementary but independent roles in the development of neural circuits. Recent evidence, however, suggests that these two mechanisms could interact extensively, with spontaneous activity affecting the expression and function of guidance molecules at early developmental stages. Here, using the developing chick spinal cord and the mouse visual system to ectopically express the inwardly rectifying potassium channel Kir2.1 in individual embryonic neurons, we demonstrate that cell-intrinsic blockade of spontaneous activity in vivo does not affect neuronal identity specification, axon pathfinding, or EphA/ephrinA signaling during the development of topographic maps. However, intrinsic spontaneous activity is critical for axon branching and pruning once axonal growth cones reach their correct topographic position in the target tissues. Our experiments argue for the dissociation of spontaneous activity from hard-wired developmental programs in early phases of neural circuit formation.
Figures
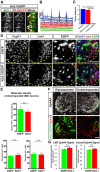
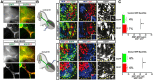
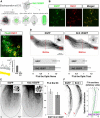

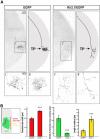
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
