Novel treatment with neuroprotective and antiviral properties against a neuroinvasive human respiratory virus
- PMID: 24227863
- PMCID: PMC3911624
- DOI: 10.1128/JVI.02972-13
Novel treatment with neuroprotective and antiviral properties against a neuroinvasive human respiratory virus
Abstract
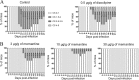
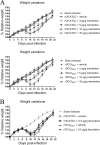
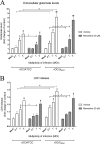
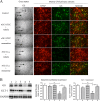
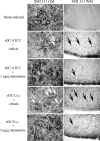
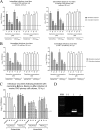
Human coronaviruses (HCoVs) are recognized respiratory pathogens with neuroinvasive and neurotropic properties in mice and humans. HCoV strain OC43 (HCoV-OC43) can infect and persist in human neural cells and activate neuroinflammatory and neurodegenerative mechanisms, suggesting that it could be involved in neurological disease of unknown etiology in humans. Moreover, we have shown that HCoV-OC43 is neurovirulent in susceptible mice, causing encephalitis, and that a viral mutant with a single point mutation in the viral surface spike (S) protein induces a paralytic disease that involves glutamate excitotoxicity in susceptible mice. Herein, we show that glutamate recycling via the glial transporter 1 protein transporter and glutamine synthetase are central to the dysregulation of glutamate homeostasis and development of motor dysfunctions and paralytic disease in HCoV-OC43-infected mice. Moreover, memantine, an N-methyl-d-aspartate receptor antagonist widely used in the treatment of neurological diseases in humans, improved clinical scores related to paralytic disease and motor disabilities by partially restoring the physiological neurofilament phosphorylation state in virus-infected mice. Interestingly, memantine attenuated mortality rates and body weight loss and reduced HCoV-OC43 replication in the central nervous system in a dose-dependent manner. This novel action of memantine on viral replication strongly suggests that it could be used as an antiviral agent to directly limit viral replication while improving neurological symptoms in various neurological diseases with a viral involvement. Mutations in the surface spike (S) protein of human respiratory coronavirus OC43 appear after persistent infection of human cells of the central nervous system, a possible viral adaptation to this environment. Furthermore, a single amino acid change in the viral S protein modulated virus-induced neuropathology in mice from an encephalitis to a neuropathology characterized by flaccid paralysis, which involves glutamate excitotoxicity. We now show that memantine, a drug that is used for alleviating symptoms associated with neuropathology, such as Alzheimer's disease, can partially restore the physiological state of infected mice by limiting both neurodegeneration and viral replication. This suggests that memantine could be used as an antiviral agent while improving neurological symptoms in various neurological diseases with a viral involvement.
Figures



References
-
- Talbot PJ, Jacomy H, Desforges M. 2008. Pathogenesis of human coronaviruses other than severe acute respiratory syndrome coronavirus, p 313–324 In Perlman S, Gallagher T, Snilder EJ. (ed), The nidoviruses. ASM Press, Washington, DC
-
- Rota PA, Oberste MS, Monroe SS, Nix WA, Campagnoli R, Icenogle JP, Penaranda S, Bankamp B, Maher K, Chen MH, Tong S, Tamin A, Lowe L, Frace M, DeRisi JL, Chen Q, Wang D, Erdman DD, Peret TC, Burns C, Ksiazek TG, Rollin PE, Sanchez A, Liffick S, Holloway B, Limor J, McCaustland K, Olsen-Rasmussen M, Fouchier R, Gunther S, Osterhaus AD, Drosten C, Pallansch MA, Anderson LJ, Bellini WJ. 2003. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 300:1394–1399. 10.1126/science.1085952 - DOI - PubMed

