ARP2/3-mediated junction-associated lamellipodia control VE-cadherin-based cell junction dynamics and maintain monolayer integrity
- PMID: 24227887
- PMCID: PMC3890345
- DOI: 10.1091/mbc.E13-07-0404
ARP2/3-mediated junction-associated lamellipodia control VE-cadherin-based cell junction dynamics and maintain monolayer integrity
Abstract
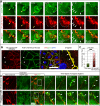
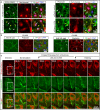
Maintenance and remodeling of endothelial cell junctions critically depend on the VE-cadherin/catenin complex and its interaction with the actin filament cytoskeleton. Here we demonstrate that local lack of vascular endothelial (VE)-cadherin at established cell junctions causes actin-driven and actin-related protein 2/3 complex (ARP2/3)-controlled lamellipodia to appear intermittently at those sites. Lamellipodia overlap the VE-cadherin-free adjacent plasma membranes and facilitate formation of new VE-cadherin adhesion sites, which quickly move into the junctions, driving VE-cadherin dynamics and remodeling. Inhibition of the ARP2/3 complex by expression of the N-WASP (V)CA domain or application of two ARP2/3 inhibitors, CK-548 and CK-666, blocks VE-cadherin dynamics and causes intercellular gaps. Furthermore, expression of carboxy-terminal-truncated VE-cadherin increases the number of ARP2/3-controlled lamellipodia, whereas overexpression of wild-type VE-cadherin largely blocks it and decreases cell motility. The data demonstrate a functional interrelationship between VE-cadherin-mediated cell adhesion and actin-driven, ARP2/3-controlled formation of new VE-cadherin adhesion sites via intermittently appearing lamellipodia at established cell junctions. This coordinated mechanism controls VE-cadherin dynamics and cell motility and maintains monolayer integrity, thus potentially being relevant in disease and angiogenesis.
Figures



References
-
- Beckers CM, van Hinsbergh VW, van Nieuw Amerongen GP. Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb Haemost. 2010;103:40–55. - PubMed
-
- Brieher WM, Yap AS. Cadherin junctions and their cytoskeleton(s) Curr Opin Cell Biol. 2013;25:39–46. - PubMed
-
- Carramusa L, Ballestrem C, Zilberman Y, Bershadsky AD. Mammalian diaphanous-related formin Dia1 controls the organization of E-cadherin-mediated cell-cell junctions. J Cell Sci. 2007;120:3870–3882. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

