Apelin-13 induces proliferation, migration, and collagen I mRNA expression in human RPE cells via PI3K/Akt and MEK/Erk signaling pathways
- PMID: 24227918
- PMCID: PMC3820432
Apelin-13 induces proliferation, migration, and collagen I mRNA expression in human RPE cells via PI3K/Akt and MEK/Erk signaling pathways
Abstract
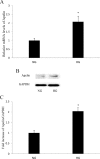
Purpose: Our previous study showed that apelin was increased in the vitreous and fibrotic membranes of patients with proliferative diabetic retinopathy (PDR) in vivo, which suggested that apelin may be involved in the development of PDR. In this study, we investigated whether the expression of apelin was upregulated in human retinal pigment epithelial (RPE) cells in vitro under high glucose conditions. Furthermore, to explore the role of apelin in RPE cells, we investigated the effect of exogenous recombinant apelin on proliferation, migration, and collagen I (a major component of extracellular matrix molecules, associated with PDR) expression and investigated the signaling pathways involved in these processes.
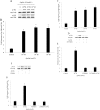
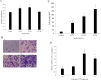
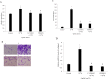
Methods: Real-time PCR and western blot were performed to determine the apelin expression in ARPE-19 cells under high glucose conditions. Exogenous recombinant apelin was used to study the effect of apelin on ARPE-19 cells in vitro. Cell proliferation, migration, and collagen I expression were assessed using an MTT assay, a transwell assay, and real-time PCR analysis. LY294002 (an inhibitor of phosphatidylinositol 3-kinase) and PD98059 (an inhibitor of mitogen-activated protein kinase) were used to help to determine the apelin signaling mechanism.
Results: High glucose upregulated apelin expression in RPE cells. Exogenous recombinant apelin activated protein kinase B (Akt) and extracellular signal-regulated kinase (Erk) phosphorylation and promoted proliferation, migration, and collagen I expression in RPE cells. Pretreatment with LY294002 and PD98059 abolished apelin-induced activation of Akt and Erk, proliferation, and collagen I expression. Apelin-induced migration was partially blocked by pretreatment with LY294002 and PD98059.
Conclusions: The expression of apelin was upregulated under high glucose conditions in RPE cells in vitro. Exogenous recombinant apelin increased the biologic activity of RPE cells, as well as the expression of collagen I. Apelin promoted proliferation, migration, and collagen I expression through the PI3K/Akt and MEK/Erk signaling pathways in RPE cells. From these results, we revealed the role of apelin in regulating proliferation, migration, and collagen I expression in RPE cells and the signaling mechanism under these processes, which suggested that apelin may play a profibrotic role in the development of PDR.
Figures
Similar articles
-
Apelin-13 Is an Early Promoter of Cytoskeleton and Tight Junction in Diabetic Macular Edema via PI-3K/Akt and MAPK/Erk Signaling Pathways.Biomed Res Int. 2018 Apr 4;2018:3242574. doi: 10.1155/2018/3242574. eCollection 2018. Biomed Res Int. 2018. PMID: 29850504 Free PMC article.
-
Protein tyrosine phosphatase 1B regulates the activity of retinal pigment epithelial cells.Mol Vis. 2015 May 1;21:523-31. eCollection 2015. Mol Vis. 2015. PMID: 25999679 Free PMC article.
-
The role of SLIT-ROBO signaling in proliferative diabetic retinopathy and retinal pigment epithelial cells.Mol Vis. 2011;17:1526-36. Epub 2011 Jun 8. Mol Vis. 2011. PMID: 21686327 Free PMC article.
-
Crosstalk between circRNAs and the PI3K/AKT and/or MEK/ERK signaling pathways in digestive tract malignancy progression.Future Oncol. 2022 Dec;18(40):4525-4538. doi: 10.2217/fon-2022-0429. Epub 2023 Mar 9. Future Oncol. 2022. PMID: 36891896 Review.
-
Targeting PI3K/AKT and MEK/ERK pathways for synergic effects on improving features of peripheral diabetic neuropathy.J Diabetes Investig. 2024 Nov;15(11):1537-1544. doi: 10.1111/jdi.14289. Epub 2024 Aug 20. J Diabetes Investig. 2024. PMID: 39162579 Free PMC article. Review.
Cited by
-
Mechanistic insights into the alterations and regulation of the AKT signaling pathway in diabetic retinopathy.Cell Death Discov. 2023 Nov 17;9(1):418. doi: 10.1038/s41420-023-01717-2. Cell Death Discov. 2023. PMID: 37978169 Free PMC article. Review.
-
Protective effect of Apelin-13 on oxygen and glucose deprivation induced-damage in retinal ganglion cells cultured in vitro.J Mol Histol. 2024 Dec 4;56(1):25. doi: 10.1007/s10735-024-10279-1. J Mol Histol. 2024. PMID: 39627585
-
Apelin promotes blood and lymph vessel formation and the growth of melanoma lung metastasis.Sci Rep. 2021 Mar 11;11(1):5798. doi: 10.1038/s41598-021-85162-0. Sci Rep. 2021. PMID: 33707612 Free PMC article.
-
Apelin-13 Is an Early Promoter of Cytoskeleton and Tight Junction in Diabetic Macular Edema via PI-3K/Akt and MAPK/Erk Signaling Pathways.Biomed Res Int. 2018 Apr 4;2018:3242574. doi: 10.1155/2018/3242574. eCollection 2018. Biomed Res Int. 2018. PMID: 29850504 Free PMC article.
-
Apelin Protects Primary Rat Retinal Pericytes from Chemical Hypoxia-Induced Apoptosis.J Ophthalmol. 2015;2015:186946. doi: 10.1155/2015/186946. Epub 2015 Sep 27. J Ophthalmol. 2015. PMID: 26491547 Free PMC article.
References
-
- Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Nakamura M. New insights into the pathophysiology of diabetic retinopathy: potential cell-specific therapeutic targets. Diabetes Technol Ther. 2000;2:601–8. - PubMed
-
- Nowak JZ. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep. 2006;58:353–63. - PubMed
-
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–81. - PubMed
-
- Abe T, Durlu YK, Tamai M. The properties of retinal pigment epithelial cells in proliferative vitreoretinopathy compared with cultured retinal pigment epithelial cells. Exp Eye Res. 1996;63:201–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
