Electrical stimulation for drug-resistant epilepsy: an evidence-based analysis
- PMID: 24228081
- PMCID: PMC3817921
Electrical stimulation for drug-resistant epilepsy: an evidence-based analysis
Abstract
Objective: The objective of this analysis was to evaluate the effectiveness of deep brain stimulation (DBS) and vagus nerve stimulation (VNS) for the treatment of drug-resistant epilepsy in adults and children.
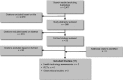
Data sources: A literature search was performed using MEDLINE, EMBASE, the Cochrane Library, and the Centre for Reviews and Dissemination database, for studies published from January 2007 until December 2012.
Review methods: Systematic reviews, meta-analyses, randomized controlled trials (RCTs), and observational studies (in the absence of RCTs) of adults or children were included. DBS studies were included if they specified that the anterior nucleus of thalamus was the area of the brain stimulated. Outcomes of interest were seizure frequency, health resource utilization, and safety. A cost analysis was also performed.
Results: The search identified 6 studies that assessed changes in seizure frequency after electrical stimulation: 1 RCT on DBS in adults, 4 RCTs on VNS in adults, and 1 RCT on VNS in children. The studies of DBS and VNS in adults found significantly improved rates of seizure frequency, but the study of VNS in children did not find a significant difference in seizure frequency between the high and low stimulation groups. Significant reductions in hospitalizations and emergency department visits were found for adults and children who received VNS. No studies addressed the use of health resources for patients undergoing DBS. Five studies reported on adverse events, which ranged from serious to transient for both procedures in adults and were mostly transient in the 1 study of VNS in children.
Limitations: We found no evidence on DBS in children or on health care use related to DBS. The measurement of seizure frequency is self-reported and is therefore subject to bias and issues of compliance.
Conclusions: Based on evidence of low to moderate quality, both DBS and VNS seemed to reduce seizure frequency in adults. In children, VNS did not appear to be as effective at reducing seizure frequency, but children had significantly fewer hospitalizations and ED visits after VNS implantation. Despite the considerable risks associated with these invasive procedures, long-term adverse events appear to be limited.
Plain language summary: Electrical stimulation of specific areas of the brain is a procedure used to control epileptic seizures when more conventional treatments are not working. Most adults and children with epilepsy are able to control their seizures with medication, but for some patients, drugs are not effective and surgery to remove the part of the brain where the seizures start is not an appropriate option. This study looked at the research available on the effectiveness, safety, and cost of two types of electrical stimulation devices currently licensed for treatment of epilepsy for adults and children in Canada: vagus nerve stimulation (VNS) and deep brain stimulation (DBS). Both approaches appear to be effective at reducing the frequency of seizures in adults. However, the evidence on DBS is limited to a single study with adults; we found no studies of DBS with children. Studies on VNS showed that both adults and children had fewer hospitalizations and emergency department visits after the procedure. Both procedures carry serious risks, but several longer-term studies have found that adverse events appear to be limited. The cost of VNS, including the process of assessing whether or not patients are good candidates for the procedure, is estimated to be about $40,000 per person (and higher for DBS because the device is more expensive and the operating time is longer). Of the 70,000 people in Ontario with epilepsy, about 1,400 (300 children and 1,110 adults) may be candidates for VNS to reduce their seizures.
Figures
References
-
- Ontario Health Technology Advisory Committee. OHTAC recommendation: care for drug-refractory epilepsy in Ontario. Ont Health Technol Assess Ser [Internet]. 2012;12(17) Available from:http://www.hqontario.ca/en/documents/eds/2012/EpilepsyOHTACRec2012.pdf .
-
- National Institute for Health and Clinical Excellence. Deep brain stimulation for refractory epilepsy [Internet]. London, UK: NHS. 2012. NICE interventional procedure guidance 416. Available from: http://guidance.nice.org.uk/IPG416 .
-
- Medical Services Advisory Committee. Vagus nerve stimulation for epilepsy [Internet]. Canberra (AU): Commonwealth of Australia. 2008. p. 115. Available from:http://www.msac.gov.au/internet/msac/publishing.nsf/Content/115CC907F004... .
-
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epi. 2011;64(4):380–2. - PubMed
-
- Goodman C. Literature searching and evidence interpretation for assessing health care practices. Stockholm: Swedish Council on Technology Assessment in Health Care. 1996:81. SBU Report No. 119F. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical