IL-18-based combinatorial adjuvants promote the intranodal production of CCL19 by NK cells and dendritic cells of cancer patients
- PMID: 24228233
- PMCID: PMC3820810
- DOI: 10.4161/onci.26245
IL-18-based combinatorial adjuvants promote the intranodal production of CCL19 by NK cells and dendritic cells of cancer patients
Abstract
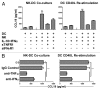
The effective accumulation and interaction of mature dendritic cells (DCs) and naïve T cells within lymph nodes (LNs), which are driven by the CCR7-CCL19/CCL21 chemokine axis, are critical for the induction of adaptive T-cell immunity. Human natural killer (NK) cells activated by interleukin (IL)-18 exhibit a unique 'helper' activity in promoting productive DC-T cell interactions, inducing DC maturation and shifting DC-primed T-cell responses toward a TH1 polarization. Here, we demonstrate that such IL-18-activated 'helper' NK cells uniquely stimulate DCs to produce high levels of CCL19 through tumor necrosis factor α (TNFα) and interferon γ (IFNγ), a process that relies on secondary NK-cell activation by additional inflammatory signals including IFNα, IL-15, IL-12 and IL-2. DCs activated by helper NK cells not only promote the efficient CCR7-mediated recruitment of naïve CD8+ T cells, but also stimulate their expansion and expression of granzyme B. Using an ex vivo explant culture system based on LNs isolated from colorectal cancer patients, we found that CCL19 is upregulated in human tumor-associated lymphoid tissues treated with helper NK cell-stimulating factors. Our findings demonstrate the ability of 2 signal-activated helper NK cells to promote the production of the DC- and naïve/memory T cell-attracting chemokine CCL19 in LNs, and provide a rationale for the therapeutic application of IL-18-containing 'combinatorial adjuvants' to facilitate the induction of antitumor immune responses.
Keywords: CCL19; IL-18; adjuvant; colorectal cancer; dendritic cell; lymph node; natural killer cell.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
