Basics and recent advances in peptide and protein drug delivery
- PMID: 24228993
- PMCID: PMC3956587
- DOI: 10.4155/tde.13.104
Basics and recent advances in peptide and protein drug delivery
Abstract
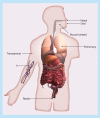
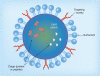
While the peptide and protein therapeutic market has developed significantly in the past decades, delivery has limited their use. Although oral delivery is preferred, most are currently delivered intravenously or subcutaneously due to degradation and limited absorption in the gastrointestinal tract. Therefore, absorption enhancers, enzyme inhibitors, carrier systems and stability enhancers are being studied to facilitate oral peptide delivery. Additionally, transdermal peptide delivery avoids the issues of the gastrointestinal tract, but also faces absorption limitations. Due to proteases, opsonization and agglutination, free peptides are not systemically stable without modifications. This review discusses oral and transdermal peptide drug delivery, focusing on barriers and solutions to absorption and stability issues. Methods to increase systemic stability and site-specific delivery are also discussed.
Figures


References
-
- Craik DJ, Fairlie DP, Liras S, Price D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013;81(1):136–147. - PubMed
-
- Department of health and human services . US FDA. Background document for meeting of advisory committee for reproductive health drugs and drug safety and risk management advisory committee. Vol. 113. MD, USA: 2013.
-
- Novartis . Novartis Annual Report. Basel, Switzerland: 2013.
-
- Zacks Investment Research; Novartis AG . Brokerage Research Digest. IL, USA: 2013.
-
- Mitchell M. The medicines company reports full year and fourth quarter 2011 financial results. NJ, USA: 2012.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
