The link between chronic periodontitis and COPD: a common role for the neutrophil?
- PMID: 24229090
- PMCID: PMC4225606
- DOI: 10.1186/1741-7015-11-241
The link between chronic periodontitis and COPD: a common role for the neutrophil?
Abstract
Background: The possible relationship between chronic inflammatory diseases and their co-morbidities has become an increasing focus of research. Both chronic periodontitis and chronic obstructive pulmonary disease are neutrophilic, inflammatory conditions characterized by the loss of local connective tissue. Evidence suggests an association and perhaps a causal link between the two diseases. However, the nature of any relationship between them is unclear, but if pathophysiologically established may have wide-reaching implications for targeted treatments to improve outcomes and prognosis.
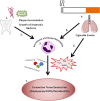
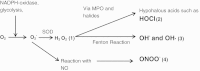
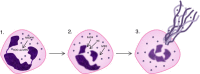
Discussion: There have been a number of epidemiological studies undertaken demonstrating an independent association between chronic periodontitis and chronic obstructive pulmonary disease. However, many of them have significant limitations, and drawing firm conclusions regarding causality may be premature. Although the pathology of both these diseases is complex and involves many cell types, such as CD8 positive cells and macrophages, both conditions are predominantly characterized by neutrophilic inflammation. Increasingly, there is evidence that the two conditions are underpinned by similar pathophysiological processes, especially centered on the functions of the neutrophil. These include a disturbance in protease/anti-protease and redox state balance. The association demonstrated by epidemiological studies, as well as emerging similarities in pathogenesis at the level of the neutrophil, suggest a basis for testing the effects of treatment for one condition upon the severity of the other.
Summary: Although the evidence of an independent association between chronic periodontitis and chronic obstructive pulmonary disease grows stronger, there remains a lack of definitive studies designed to establish causality and treatment effects. There is a need for future research to be focused on answering these questions.
Figures

References
-
- Morris AJ, Steele J, White DA. The oral cleanliness and periodontal health of UK adults in 1998. Br Dent J. 2001;191:186–192. - PubMed
-
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D. et al.Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global burden of disease study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. - DOI - PMC - PubMed
-
- Kinane DF, Preshaw PM, Loos BG. Host-response: understanding the cellular and molecular mechanisms of host-microbial interactions–consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011;38:44–48. - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

