CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis
- PMID: 24229710
- PMCID: PMC3928012
- DOI: 10.1016/j.ccr.2013.10.009
CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis
Abstract
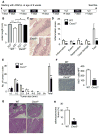
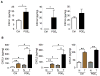
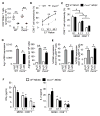
A large body of evidence indicates that chronic inflammation is one of several key risk factors for cancer initiation, progression, and metastasis. However, the underlying mechanisms responsible for the contribution of inflammation and inflammatory mediators to cancer remain elusive. Here, we present genetic evidence that loss of CXCR2 dramatically suppresses chronic colonic inflammation and colitis-associated tumorigenesis through inhibiting infiltration of myeloid-derived suppressor cells (MDSCs) into colonic mucosa and tumors in a mouse model of colitis-associated cancer. CXCR2 ligands were elevated in inflamed colonic mucosa and tumors and induced MDSC chemotaxis. Adoptive transfer of wild-type MDSCs into Cxcr2(-/-) mice restored AOM/DSS-induced tumor progression. MDSCs accelerated tumor growth by inhibiting CD8(+) T cell cytotoxic activity.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

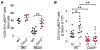


References
-
- Abdallah Hajj Hussein I, Freund JN, Reimund JM, Shams A, Yamine M, Leone A, Jurjus AR. Enteropathogenic e.coli sustains iodoacetamide-induced ulcerative colitis-like colitis in rats: modulation of IL-1beta, IL-6, TNF-alpha, COX-2, and apoptosisi. Journal of biological regulators and homeostatic agents. 2012;26:515–526. - PubMed
-
- Alzoghaibi MA, Al-Mofleh IA, Al-Jebreen AM. Neutrophil chemokines GCP-2 and GRO-alpha in patients with inflammatory bowel disease. J Dig Dis. 2008;9:144–148. - PubMed
-
- Andres PG, Beck PL, Mizoguchi E, Mizoguchi A, Bhan AK, Dawson T, Kuziel WA, Maeda N, MacDermott RP, Podolsky DK, Reinecker HC. Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J Immunol. 2000;164:6303–6312. - PubMed
-
- Asfaha S, Dubeykovskiy AN, Tomita H, Yang X, Stokes S, Shibata W, Friedman RA, Ariyama H, Dubeykovskaya ZA, Muthupalani S, et al. Mice that express human interleukin-8 have increased mobilization of immature myeloid cells, which exacerbates inflammation and accelerates colon carcinogenesis. Gastroenterology. 2013;144:155–166. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

