Comprehensive control of human papillomavirus infections and related diseases
- PMID: 24229716
- PMCID: PMC4062073
- DOI: 10.1016/j.vaccine.2013.07.026
Comprehensive control of human papillomavirus infections and related diseases
Abstract
Infection with human papillomavirus (HPV) is recognized as one of the major causes of infection-related cancer worldwide, as well as the causal factor in other diseases. Strong evidence for a causal etiology with HPV has been stated by the International Agency for Research on Cancer for cancers of the cervix uteri, penis, vulva, vagina, anus and oropharynx (including base of the tongue and tonsils). Of the estimated 12.7 million new cancers occurring in 2008 worldwide, 4.8% were attributable to HPV infection, with substantially higher incidence and mortality rates seen in developing versus developed countries. In recent years, we have gained tremendous knowledge about HPVs and their interactions with host cells, tissues and the immune system; have validated and implemented strategies for safe and efficacious prophylactic vaccination against HPV infections; have developed increasingly sensitive and specific molecular diagnostic tools for HPV detection for use in cervical cancer screening; and have substantially increased global awareness of HPV and its many associated diseases in women, men, and children. While these achievements exemplify the success of biomedical research in generating important public health interventions, they also generate new and daunting challenges: costs of HPV prevention and medical care, the implementation of what is technically possible, socio-political resistance to prevention opportunities, and the very wide ranges of national economic capabilities and health care systems. Gains and challenges faced in the quest for comprehensive control of HPV infection and HPV-related cancers and other disease are summarized in this review. The information presented may be viewed in terms of a reframed paradigm of prevention of cervical cancer and other HPV-related diseases that will include strategic combinations of at least four major components: 1) routine introduction of HPV vaccines to women in all countries, 2) extension and simplification of existing screening programs using HPV-based technology, 3) extension of adapted screening programs to developing populations, and 4) consideration of the broader spectrum of cancers and other diseases preventable by HPV vaccination in women, as well as in men. Despite the huge advances already achieved, there must be ongoing efforts including international advocacy to achieve widespread-optimally universal-implementation of HPV prevention strategies in both developed and developing countries. This article summarizes information from the chapters presented in a special ICO Monograph 'Comprehensive Control of HPV Infections and Related Diseases' Vaccine Volume 30, Supplement 5, 2012. Additional details on each subtopic and full information regarding the supporting literature references may be found in the original chapters.
Keywords: Anal cancer; Cervical cancer; HPV; HPV testing; HPV vaccination; Oropharyngeal cancer; Penile cancer; Prevention; Screening; Vaginal cancer; Vulvar cancer.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
FXB: Has received occasional travel grants to conferences/symposia/meetings and honorarium by either GlaxoSmithKline, Merck, Sanofi Pasteur MSD, Roche or Qiagen. The Unit of Infections and Cancer at the ICO is involved in HPV vaccine trials and epidemiological studies sponsored by GlaxoSmithKline, Merck and Sanofi Pasteur MSD.
TRB: Has disclosed no potential conflicts of interest.
DF: Has disclosed no potential conflicts of interest.
ABM: serves as an advisory board member for Merck and receives support for sexually transmitted disease testing from Gen-Probe. She has also received travel funds and an honorarium for speaking at a symposium supported by Becton Dickinson.
MLG: Has had scientific collaborations and has received research funding from Merck. She has acted as a consultant for Merck and GlaxoSmithKline.
JD: Is supported by the UK Medical Research Council, has recently acted as consultant for Sanofi Pasteur MSD, Merck and Roche, and has received research support from Sanofi Pasteur MSD, GlaxoSmithKline and the Wellcome Trust.
PLS: Has received support for Travel, Lectureships, (GlaxoSmithKline); Consultancy (GlaxoSmithKline, Oxford Biomedica); Meeting/Travel expenses (GlaxoSmithKline, Oxford Biomedica).
M. Stanley: Has acted as a consultant for MSD Merck, Sanofi Pasteur MSD and GlaxoSmithKline.
MA: Has disclosed no potential conflicts of interest.
MP: Advisory Board (Roche); Consultant (Abbott); Research Grants (Abbott, Roche); Speakers Bureau (Abbott, Roche); Travel Grants (Abbott, Roche).
JC: Is on advisory Boards for Abbott, Becton Dickinson, Gen-Probe, Qiagen and Roche and his institution receives research funding from these companies as well as Genera Biosystems and Oncohealth.
PEC: Serves as a member of a Data and Safety Monitoring Board to review data on HPV vaccines for Merck, has received HPV test reagents and testing for research from Qiagen and Roche at a reduced or no cost, and has consulted for BD, Gen-Probe/Hologic, Roche, Cepheid, and GE Healthcare.
JTS: Named inventor on U.S. government-owned HPV vaccine-related patents that are licensed to Merck & Co., GlaxoSmithKline, Sanofi Pasteur and Shantha Biotechnics and is entitled to limited royalties as specified by federal law.
LEM: Has disclosed no potential conflicts of interest.
WAF: Has received speaker fees, and educational and unrestricted research grants from Merck Canada.
KC: Is co-PI of a new trial of primary HPV screening in Australia, which is partially supported by Roche Molecular Diagnostics USA.
LAD: Has received honoraria from GlaxoSmithKline and Merck for appearing on various speaker fora and sponsorship for research studies.
ELF: Has served as occasional consultant to companies involved with HPV vaccines (Merck and GlaxoSmithKline) and with HPV diagnostics (Roche, Gen-Probe, Becton Dickinson). He has received an unrestricted grant from Merck.
M. Steben: Consultant (Roche Molecular, Digene and Graceway Pharma); Research Grants (Merck and GlaxoSmithKline); Speakers Bureaus (Merck, Graceway Pharma, Digene and Laboratoire Biron); Educational presentations (Merck and Graceway Pharma); Payment for manuscript preparation (Graceway Pharma).
MAK: Member of the Merck Advisory Board for HPV vaccine and has received consulting fees, honoraria and travel support from Merck.
M. Schiffman: Has received free CareHPV equipment and reagents for an independent analysis of low-cost HPV testing in Africa. He has also received free specimen testing from Roche for epidemiologic projects. He was the NCI co-Project Officer and co-Medical Monitor of an independent evaluation of GlaxoSmithKline HPV vaccine, for which GlaxoSmithKline donated vaccine and financed the regulatory components.
CJLMM: Scientific advisory board (Qiagen); Speakers office (GlaxoSmithKline, Roche); Research Grant (Abbott); Shares minority (Self-Screen B.V).
RS: Has disclosed no potential conflicts of interest.
XC: Has received occasional travel grants to scientific meetings and honorarium for consultancy by either GlaxoSmithKline, Merck, Sanofi Pasteur MSD. The Unit of Infections and Cancer at the ICO is involved in HPV vaccine trials and epidemiological studies sponsored by GlaxoSmithKline, Merck and Sanofi Pasteur MSD.
JJK: Has disclosed no potential conflicts of interest.
MB: Has received occasional travel grants to conferences by GlaxoSmithKline or Sanofi Pasteur MSD. The Unit of Infections and Cancer at the ICO is involved in HPV vaccine trials and epidemiological studies sponsored by GlaxoSmithKline, Merck and Sanofi Pasteur MSD.
LA: Has received occasional travel grants to conferences by Merck and Sanofi Pasteur MSD. The Unit of Infections and Cancer at the ICO is involved in HPV vaccine trials and epidemiological studies sponsored by GlaxoSmithKline, Merck and Sanofi Pasteur MSD.
GA: Has received occasional travel grants to conferences/meetings by either GlaxoSmithKline, Merck, Sanofi Pasteur MSD, Roche or Qiagen. The Unit of Infections and Cancer at the ICO is involved in HPV vaccine trials and epidemiological studies sponsored by GlaxoSmithKline, Merck and Sanofi Pasteur MSD.
MD: Has disclosed no potential conflicts of interest. The Unit of Infections and Cancer at the ICO is involved in HPV vaccine trials and epidemiological studies sponsored by GlaxoSmithKline, Merck and Sanofi Pasteur MSD.
SdS: Has received occasional travel grants to conferences/symposia/meetings by either GlaxoSmithKline, Sanofi Pasteur MSD or Qiagen. The Unit of Infections and Cancer at the ICO is involved in HPV vaccine trials and epidemiological studies sponsored by GlaxoSmithKline, Merck and Sanofi Pasteur MSD.
Figures

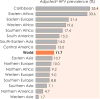

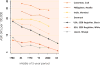





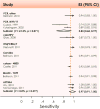




Republished in
-
Comprehensive control of human papillomavirus infections and related diseases.Vaccine. 2013 Dec 30;31 Suppl 6:G1-31. doi: 10.1016/j.vaccine.2013.10.002. Vaccine. 2013. PMID: 24331817 Review.
-
Comprehensive control of human papillomavirus infections and related diseases.Vaccine. 2013 Dec 31;31 Suppl 7(Suppl 7):H1-31. doi: 10.1016/j.vaccine.2013.10.003. Vaccine. 2013. PMID: 24332295 Free PMC article.
References
-
- Broker TR. Global prevention and management of human papillomavirus related diseases: the pressing challenges and the compelling opportunities. Vaccine. 2012;30(S5):7–10. - PubMed
-
- Bosch FX, Tsu V, Vorsters A, Van Damme P, Kane MA. Reframing cervical cancer prevention. Expanding the field towards prevention of human papillomavirus infections and related diseases Vaccine. 2012;30(S5):F1–11. - PubMed
-
- Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(S5):F12–23. - PubMed
-
- Gillison ML, Alemany L, Snijders PJF, Chaturvedi A, Steinberg BM, Schwartz S, et al. Human papillomavirus and diseases of the upper airway: head and neck cancer and respiratory papillomatosis. Vaccine. 2012;30(S5):F34–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RC1 AI086051/AI/NIAID NIH HHS/United States
- R01 CA160744/CA/NCI NIH HHS/United States
- R01CA160744-01A1/CA/NCI NIH HHS/United States
- R01 CA083679/CA/NCI NIH HHS/United States
- RC1AI86051/AI/NIAID NIH HHS/United States
- U54CA164336/CA/NCI NIH HHS/United States
- CA83679/CA/NCI NIH HHS/United States
- R37CA51323/CA/NCI NIH HHS/United States
- MC_PC_13050/MRC_/Medical Research Council/United Kingdom
- U54 CA164336/CA/NCI NIH HHS/United States
- R37 CA051323/CA/NCI NIH HHS/United States
- MC_U117584278/MRC_/Medical Research Council/United Kingdom
- MC U117584278/MRC_/Medical Research Council/United Kingdom
- CA36200/CA/NCI NIH HHS/United States
- R01 CA036200/CA/NCI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

