Microvesicles as mediators of tissue regeneration
- PMID: 24231336
- PMCID: PMC3976717
- DOI: 10.1016/j.trsl.2013.10.005
Microvesicles as mediators of tissue regeneration
Abstract
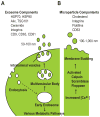
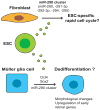
The use of stem cells in the treatment of various diseases and injuries has received increasing interest during the past decade. Injected stem cells, such as mesenchymal stem cells, stimulate tissue repair largely through the secretion of soluble factors that regulate various processes of tissue regeneration, including inflammatory responses, apoptosis, host cell proliferation, and angiogenesis. Recently, it has become apparent that stem cells also use membranous small vesicles, collectively called microvesicles, to repair damaged tissues. Microvesicles are released by many types of cells and exist in almost all types of body fluids. They serve as a vehicle to transfer protein, messenger RNA, and micro RNA to distant cells, altering the gene expression, proliferation, and differentiation of the recipient cells. Although animal models and in vitro studies have suggested promising applications for microvesicles-based regeneration therapy, its effectiveness and feasibility in clinical medicine remain to be established. Further studies of the basic mechanisms responsible for microvesicle-mediated tissue regeneration could lead to novel approaches in regenerative medicine.
Copyright © 2014 Mosby, Inc. All rights reserved.
Figures


Similar articles
-
Progress with stem cell therapies for tendon tissue regeneration.Expert Opin Biol Ther. 2020 Nov;20(11):1373-1379. doi: 10.1080/14712598.2020.1786532. Epub 2020 Jun 29. Expert Opin Biol Ther. 2020. PMID: 32574078 Review.
-
Recent Approaches for Angiogenesis in Search of Successful Tissue Engineering and Regeneration.Curr Stem Cell Res Ther. 2020;15(2):111-134. doi: 10.2174/1574888X14666191104151928. Curr Stem Cell Res Ther. 2020. PMID: 31682212 Review.
-
Tissue engineering and regenerative medicine: past, present, and future.Int Rev Neurobiol. 2013;108:1-33. doi: 10.1016/B978-0-12-410499-0.00001-0. Int Rev Neurobiol. 2013. PMID: 24083429 Review.
-
Regenerative medicine in dermatology: biomaterials, tissue engineering, stem cells, gene transfer and beyond.Exp Dermatol. 2010 Aug;19(8):697-706. doi: 10.1111/j.1600-0625.2010.01087.x. Exp Dermatol. 2010. PMID: 20545761 Review.
-
The Future of Cardiovascular Regenerative Medicine.Circulation. 2016 Jun 21;133(25):2618-25. doi: 10.1161/CIRCULATIONAHA.115.019214. Circulation. 2016. PMID: 27324357 Free PMC article. Review.
Cited by
-
Exosome: A New Player in Translational Nanomedicine.J Clin Med. 2020 Jul 26;9(8):2380. doi: 10.3390/jcm9082380. J Clin Med. 2020. PMID: 32722531 Free PMC article. Review.
-
The Effect of Mesenchymal Stem Cell-Derived Microvesicles on Erythroid Differentiation of Umbilical Cord Blood-Derived CD34+ Cells.Adv Pharm Bull. 2018 Jun;8(2):291-296. doi: 10.15171/apb.2018.034. Epub 2018 Jun 19. Adv Pharm Bull. 2018. PMID: 30023331 Free PMC article.
-
Therapeutic potential of adipose stem cell-derived conditioned medium against pulmonary hypertension and lung fibrosis.Br J Pharmacol. 2016 Oct;173(19):2859-79. doi: 10.1111/bph.13562. Epub 2016 Aug 26. Br J Pharmacol. 2016. PMID: 27448286 Free PMC article.
-
Circulating Extracellular RNA Markers of Liver Regeneration.PLoS One. 2016 Jul 14;11(7):e0155888. doi: 10.1371/journal.pone.0155888. eCollection 2016. PLoS One. 2016. PMID: 27415797 Free PMC article.
-
Mesenchymal stem cell derived secretome and extracellular vesicles for acute lung injury and other inflammatory lung diseases.Expert Opin Biol Ther. 2016 Jul;16(7):859-71. doi: 10.1517/14712598.2016.1170804. Epub 2016 Apr 12. Expert Opin Biol Ther. 2016. PMID: 27011289 Free PMC article. Review.
References
-
- Mathivanan S, Ji H, Simpson RJ. Exosomes: Extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–20. - PubMed
-
- Simons M, Raposo G. Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–81. - PubMed
-
- Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–8. - PubMed
-
- Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011;81:1171–82. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

