Systems pharmacology identifies drug targets for Stargardt disease-associated retinal degeneration
- PMID: 24231350
- PMCID: PMC3859412
- DOI: 10.1172/JCI69076
Systems pharmacology identifies drug targets for Stargardt disease-associated retinal degeneration
Abstract
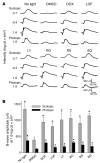
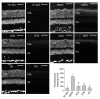
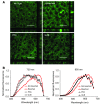
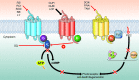
A systems pharmacological approach that capitalizes on the characterization of intracellular signaling networks can transform our understanding of human diseases and lead to therapy development. Here, we applied this strategy to identify pharmacological targets for the treatment of Stargardt disease, a severe juvenile form of macular degeneration. Diverse GPCRs have previously been implicated in neuronal cell survival, and crosstalk between GPCR signaling pathways represents an unexplored avenue for pharmacological intervention. We focused on this receptor family for potential therapeutic interventions in macular disease. Complete transcriptomes of mouse and human samples were analyzed to assess the expression of GPCRs in the retina. Focusing on adrenergic (AR) and serotonin (5-HT) receptors, we found that adrenoceptor α 2C (Adra2c) and serotonin receptor 2a (Htr2a) were the most highly expressed. Using a mouse model of Stargardt disease, we found that pharmacological interventions that targeted both GPCR signaling pathways and adenylate cyclases (ACs) improved photoreceptor cell survival, preserved photoreceptor function, and attenuated the accumulation of pathological fluorescent deposits in the retina. These findings demonstrate a strategy for the identification of new drug candidates and FDA-approved drugs for the treatment of monogenic and complex diseases.
Figures








Similar articles
-
Mechanism of all-trans-retinal toxicity with implications for stargardt disease and age-related macular degeneration.J Biol Chem. 2012 Feb 10;287(7):5059-69. doi: 10.1074/jbc.M111.315432. Epub 2011 Dec 19. J Biol Chem. 2012. PMID: 22184108 Free PMC article.
-
Complement modulation in the retinal pigment epithelium rescues photoreceptor degeneration in a mouse model of Stargardt disease.Proc Natl Acad Sci U S A. 2017 Apr 11;114(15):3987-3992. doi: 10.1073/pnas.1620299114. Epub 2017 Mar 27. Proc Natl Acad Sci U S A. 2017. PMID: 28348233 Free PMC article.
-
Activation of JNK signaling promotes all-trans-retinal-induced photoreceptor apoptosis in mice.J Biol Chem. 2020 May 15;295(20):6958-6971. doi: 10.1074/jbc.RA120.013189. Epub 2020 Apr 7. J Biol Chem. 2020. PMID: 32265302 Free PMC article.
-
Molecular pharmacology of alpha 2-adrenoceptor subtypes.Ann Med. 1995 Aug;27(4):439-49. doi: 10.3109/07853899709002452. Ann Med. 1995. PMID: 8519505 Review.
-
Defective lipid transport and biosynthesis in recessive and dominant Stargardt macular degeneration.Prog Lipid Res. 2010 Oct;49(4):476-92. doi: 10.1016/j.plipres.2010.07.002. Epub 2010 Jul 13. Prog Lipid Res. 2010. PMID: 20633576 Free PMC article. Review.
Cited by
-
Drug Discovery Strategies for Inherited Retinal Degenerations.Biology (Basel). 2022 Sep 10;11(9):1338. doi: 10.3390/biology11091338. Biology (Basel). 2022. PMID: 36138817 Free PMC article. Review.
-
Eyes on systems pharmacology.Pharmacol Res. 2016 Dec;114:39-41. doi: 10.1016/j.phrs.2016.09.026. Epub 2016 Oct 5. Pharmacol Res. 2016. PMID: 27720767 Free PMC article. No abstract available.
-
Scavenging of Cation Radicals of the Visual Cycle Retinoids by Lutein, Zeaxanthin, Taurine, and Melanin.Int J Mol Sci. 2023 Dec 29;25(1):506. doi: 10.3390/ijms25010506. Int J Mol Sci. 2023. PMID: 38203675 Free PMC article.
-
Synergistically acting agonists and antagonists of G protein-coupled receptors prevent photoreceptor cell degeneration.Sci Signal. 2016 Jul 26;9(438):ra74. doi: 10.1126/scisignal.aag0245. Sci Signal. 2016. PMID: 27460988 Free PMC article.
-
Adrenergic and serotonin receptors affect retinal superoxide generation in diabetic mice: relationship to capillary degeneration and permeability.FASEB J. 2015 May;29(5):2194-204. doi: 10.1096/fj.14-269431. Epub 2015 Feb 9. FASEB J. 2015. PMID: 25667222 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- K08 EY019031/EY/NEI NIH HHS/United States
- R01 EY009339/EY/NEI NIH HHS/United States
- P30 EY011373/EY/NEI NIH HHS/United States
- K08EY019880/EY/NEI NIH HHS/United States
- T32 EY007157/EY/NEI NIH HHS/United States
- AG043645/AG/NIA NIH HHS/United States
- T32GM007250/GM/NIGMS NIH HHS/United States
- K08EY019031/EY/NEI NIH HHS/United States
- R01 EY022658/EY/NEI NIH HHS/United States
- R44 AG043645/AG/NIA NIH HHS/United States
- EY009339/EY/NEI NIH HHS/United States
- T32EY007157/EY/NEI NIH HHS/United States
- P30 EY11373/EY/NEI NIH HHS/United States
- K08 EY019880/EY/NEI NIH HHS/United States
- R24 EY021126/EY/NEI NIH HHS/United States
- T32 GM007250/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

