Adaptive immune activation: glycosylation does matter
- PMID: 24231619
- PMCID: PMC3966069
- DOI: 10.1038/nchembio.1403
Adaptive immune activation: glycosylation does matter
Abstract
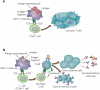
Major histocompatibility complex (MHC) class I and II are glycoproteins that can present antigenic peptides at the cell surface for recognition and activation of circulating T lymphocytes. Here, the importance of the modification of protein antigens by glycans on cellular uptake, proteolytic processing, presentation by MHC and subsequent T-cell priming is reviewed. Antigen glycosylation is important for a number of diseases and vaccine design. All of the key proteins involved in antigen recognition and the orchestration of downstream effector functions are glycosylated. The influence of protein glycosylation on immune function and disease is covered.
Figures


References
-
- Roth J. Protein N-glycosylation along the secretory pathway: relationship to organelle topography and function, protein quality control, and cell interactions. Chem. Rev. 2002;102:285–303. - PubMed
-
- Kleene R, Schachner M. Glycans and neural cell interactions. Nat. Rev. Neurosci. 2004;5:195–208. - PubMed
-
- Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. - PubMed
-
- Prescher JA, Bertozzi CR. Chemical technologies for probing glycans. Cell. 2006;126:851–854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

