Plasma apolipoprotein L1 levels do not correlate with CKD
- PMID: 24231663
- PMCID: PMC3935593
- DOI: 10.1681/ASN.2013070700
Plasma apolipoprotein L1 levels do not correlate with CKD
Abstract
Polymorphisms in APOL1 are associated with CKD, including HIV-related CKD, in individuals of African ancestry. The apolipoprotein L1 (APOL1) protein circulates and is localized in kidney cells, but the contribution of APOL1 location to CKD pathogenesis is unclear. We examined associations of plasma APOL1 levels with plasma cytokine levels, dyslipidemia, and APOL1 genotype in a nested case-control study (n=270) of HIV-infected African Americans enrolled in a multicenter prospective observational study. Patients were designated as having CKD when estimated GFR (eGFR) decreased to <60 ml/min per 1.73 m(2) (eGFR<60 cohort) or protein-to-creatinine ratios became >3.5 g/g (nephrotic proteinuria cohort). Circulating APOL1 levels did not associate with APOL1 genotype, CKD status, or levels of proinflammatory cytokines, but did correlate with fasting cholesterol, LDL cholesterol, and triglyceride levels. At ascertainment, CKD-associated polymorphisms (risk variants) in APOL1 associated with the eGFR<60 cohort, but not the nephrotic-range proteinuria cohort. Of note, in both the eGFR<60 and nephrotic proteinuria cohorts, CKD cases with two APOL1 risk variants had significant declines in eGFR over a median of 4 years compared with individuals with one or no risk variants. APOL1 risk genotype was not associated with changes in proteinuria. Higher circulating proinflammatory cytokine levels were independently associated with CKD but not APOL1 genotype. In conclusion, the function of variant APOL1 proteins derived from circulation or synthesized in the kidney, but not the level of circulating APOL1, probably mediates APOL1-associated kidney disease in HIV-infected African Americans.
Figures
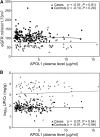

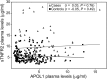
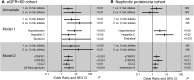

References
-
- Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 - PMC - PubMed
-
- Genovese G, Friedman DJ, Pollak MR: APOL1 variants and kidney disease in people of recent African ancestry. Nat Rev Nephrol 9: 240–244, 2013 - PubMed
-
- Ulasi II, Tzur S, Wasser WG, Shemer R, Kruzel E, Feigin E, Ijoma CK, Onodugo OD, Okoye JU, Arodiwe EB, Ifebunandu NA, Chukwuka CJ, Onyedum CC, Ijoma UN, Nna E, Onuigbo M, Rosset S, Skorecki K: High population frequencies of APOL1 risk variants are associated with increased prevalence of non-diabetic chronic kidney disease in the Igbo people from South-Eastern Nigeria. Nephron Clin Pract 123: 123–128, 2013 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UM1 AI069501/AI/NIAID NIH HHS/United States
- AI69501/AI/NIAID NIH HHS/United States
- R01 MD007092/MD/NIMHD NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- UL1TR000439/TR/NCATS NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- AI38855/AI/NIAID NIH HHS/United States
- AI036219/AI/NIAID NIH HHS/United States
- P30 AI036219/AI/NIAID NIH HHS/United States
- AI68634/AI/NIAID NIH HHS/United States
- AI68636/AI/NIAID NIH HHS/United States
- AI38858/AI/NIAID NIH HHS/United States
- U01 AI038855/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- U01 AI069501/AI/NIAID NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

