Water influx into cerebrospinal fluid is primarily controlled by aquaporin-4, not by aquaporin-1: 17O JJVCPE MRI study in knockout mice
- PMID: 24231830
- PMCID: PMC4235386
- DOI: 10.1097/WNR.0000000000000042
Water influx into cerebrospinal fluid is primarily controlled by aquaporin-4, not by aquaporin-1: 17O JJVCPE MRI study in knockout mice
Abstract
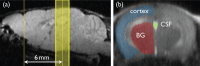
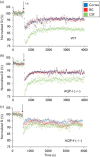
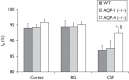
Recent studies on cerebrospinal fluid (CSF) homeostasis emphasize the importance of water flux through the pericapillary (Virchow-Robin) space for both CSF production and reabsorption (Oreskovic and Klarica hypothesis), and challenge the classic CSF circulation theory, which proposes that CSF is primarily produced by the choroid plexus and reabsorbed by the arachnoid villi. Active suppression of aquaporin-1 (AQP-1) expression within brain capillaries and preservation of AQP-1 within the choroid plexus together with pericapillary water regulation by AQP-4 provide a unique opportunity for testing this recent hypothesis. We investigated water flux into three representative regions of the brain, namely, the cortex, basal ganglia, and third ventricle using a newly developed water molecular MRI technique based on JJ vicinal coupling between O and adjacent protons and water molecule proton exchanges (JJVCPE imaging) in AQP-1 and AQP-4 knockout mice in vivo. The results clearly indicate that water influx into the CSF is regulated by AQP-4, and not by AQP-1, strongly supporting the Oreskovic and Klarica hypothesis.
Figures


References
-
- Orešković D, Klarica M.The formation of cerebrospinal fluid: nearly a hundred years of interpretations and misinterpretations.Brain Res Rev 2010;64:241–262. - PubMed
-
- Igarashi H, Tsujita M, Huber VJ, Kwee IL, Nakada T.Inhibition of aquaporin-4 significantly increases regional cerebral blood flow.NeuroReport 2013;24:324–328. - PubMed
-
- Huber VJ, Tsujita M, Nakada T.Aquaporins in drug discovery and pharmacotherapy.Mol Aspects Med 2012;33:691–703. - PubMed
-
- Dolman D, Drndarski S, Abbott NJ, Rattray M.Induction of aquaporin 1 but not aquaporin 4 messenger RNA in rat primary brain microvessel endothelial cells in culture.J Neurochem 2005;93:825–833. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

