Cellular dynamics in the muscle satellite cell niche
- PMID: 24232182
- PMCID: PMC3849491
- DOI: 10.1038/embor.2013.182
Cellular dynamics in the muscle satellite cell niche
Abstract
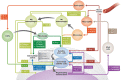
Satellite cells, the quintessential skeletal muscle stem cells, reside in a specialized local environment whose anatomy changes dynamically during tissue regeneration. The plasticity of this niche is attributable to regulation by the stem cells themselves and to a multitude of functionally diverse cell types. In particular, immune cells, fibrogenic cells, vessel-associated cells and committed and differentiated cells of the myogenic lineage have emerged as important constituents of the satellite cell niche. Here, we discuss the cellular dynamics during muscle regeneration and how disease can lead to perturbation of these mechanisms. To define the role of cellular components in the muscle stem cell niche is imperative for the development of cell-based therapies, as well as to better understand the pathobiology of degenerative conditions of the skeletal musculature.
Figures


References
-
- Lieber RL (2010) Skeletal Muscle Structure, Function, and Plasticity. Baltimore, MD, USA: Wolters Kluwer/Lippincott Williams & Wilkins
-
- Pannerec A, Marazzi G, Sassoon D (2012) Stem cells in the hood: the skeletal muscle niche. Trends Mol Med 18: 599–606 - PubMed
-
- Mounier R, Chretien F, Chazaud B (2011) Blood vessels and the satellite cell niche. Curr Top Dev Biol 96: 121–138 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

