Contralaterally transplanted human embryonic stem cell-derived neural precursor cells (ENStem-A) migrate and improve brain functions in stroke-damaged rats
- PMID: 24232252
- PMCID: PMC3849578
- DOI: 10.1038/emm.2013.93
Contralaterally transplanted human embryonic stem cell-derived neural precursor cells (ENStem-A) migrate and improve brain functions in stroke-damaged rats
Abstract
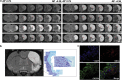
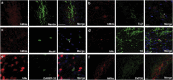
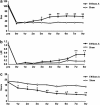
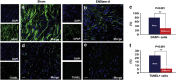
The transplantation of neural precursor cells (NPCs) is known to be a promising approach to ameliorating behavioral deficits after stroke in a rodent model of middle cerebral artery occlusion (MCAo). Previous studies have shown that transplanted NPCs migrate toward the infarct region, survive and differentiate into mature neurons to some extent. However, the spatiotemporal dynamics of NPC migration following transplantation into stroke animals have yet to be elucidated. In this study, we investigated the fates of human embryonic stem cell (hESC)-derived NPCs (ENStem-A) for 8 weeks following transplantation into the side contralateral to the infarct region using 7.0T animal magnetic resonance imaging (MRI). T2- and T2*-weighted MRI analyses indicated that the migrating cells were clearly detectable at the infarct boundary zone by 1 week, and the intensity of the MRI signals robustly increased within 4 weeks after transplantation. Afterwards, the signals were slightly increased or unchanged. At 8 weeks, we performed Prussian blue staining and immunohistochemical staining using human-specific markers, and found that high percentages of transplanted cells migrated to the infarct boundary. Most of these cells were CXCR4-positive. We also observed that the migrating cells expressed markers for various stages of neural differentiation, including Nestin, Tuj1, NeuN, TH, DARPP-32 and SV38, indicating that the transplanted cells may partially contribute to the reconstruction of the damaged neural tissues after stroke. Interestingly, we found that the extent of gliosis (glial fibrillary acidic protein-positive cells) and apoptosis (TUNEL-positive cells) were significantly decreased in the cell-transplanted group, suggesting that hESC-NPCs have a positive role in reducing glia scar formation and cell death after stroke. No tumors formed in our study. We also performed various behavioral tests, including rotarod, stepping and modified neurological severity score tests, and found that the transplanted animals exhibited significant improvements in sensorimotor functions during the 8 weeks after transplantation. Taken together, these results strongly suggest that hESC-NPCs have the capacity to migrate to the infarct region, form neural tissues efficiently and contribute to behavioral recovery in a rodent model of ischemic stroke.
Figures

References
-
- van Wijngaarden JD, Dirks M, Huijsman R, Niessen LW, Fabbricotti IN, Dippel DW. Hospital rates of thrombolysis for acute ischemic stroke: the influence of organizational culture. Stroke. 2009;40:3390–3392. - PubMed
-
- Mack GS. ReNeuron and StemCells get green light for neural stem cell trials. Nat Biotechnol. 2011;29:95–97. - PubMed
-
- Buhnemann C, Scholz A, Bernreuther C, Malik CY, Braun H, Schachner M, et al. Neuronal differentiation of transplanted embryonic stem cell-derived precursors in stroke lesions of adult rats. Brain. 2006;129:3238–3248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
