In situ tissue regeneration through host stem cell recruitment
- PMID: 24232256
- PMCID: PMC3849571
- DOI: 10.1038/emm.2013.118
In situ tissue regeneration through host stem cell recruitment
Abstract
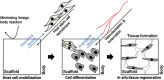
The field of tissue engineering has made steady progress in translating various tissue applications. Although the classical tissue engineering strategy, which involves the use of culture-expanded cells and scaffolds to produce a tissue construct for implantation, has been validated, this approach involves extensive cell expansion steps, requiring a lot of time and laborious effort before implantation. To bypass this ex vivo process, a new approach has been introduced. In situ tissue regeneration utilizes the body's own regenerating capacity by mobilizing host endogenous stem cells or tissue-specific progenitor cells to the site of injury. This approach relies on development of a target-specific biomaterial scaffolding system that can effectively control the host microenvironment and mobilize host stem/progenitor cells to target tissues. An appropriate microenvironment provided by implanted scaffolds would facilitate recruitment of host cells that can be guided to regenerating structural and functional tissues.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
