Metronomic capecitabine in advanced hepatocellular carcinoma patients: a phase II study
- PMID: 24232581
- PMCID: PMC3868418
- DOI: 10.1634/theoncologist.2013-0093
Metronomic capecitabine in advanced hepatocellular carcinoma patients: a phase II study
Abstract
Anti-angiogenic treatment with targeted agents is effective in advanced hepatocellular carcinoma (HCC). This trial evaluated the safety and efficacy of metronomic capecitabine in patients with HCC.
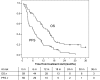
Methods: This single-institution phase II trial included 59 previously untreated patients with advanced HCC and 31 patients resistant to or intolerant of sorafenib. The treatment schedule was capecitabine 500 mg twice daily until progression of disease, unacceptable toxicity level, or withdrawal of informed consent. Progression-free survival (PFS) was chosen as the primary endpoint.
Results: A total of 59 previously untreated and 31 previously treated patients with HCC were enrolled. The first cohort achieved a median PFS of 6.03 months and an overall survival (OS) of 14.47 months. Two patients achieved a complete response, 1 patient achieved partial response, and in 30 patients, stable disease was the best outcome. The second cohort achieved a median PFS of 3.27 months and a median OS of 9.77 months. No complete or partial responses were observed, but 10 patients had stable disease. An unscheduled comparison of the first cohort of patients with 3,027 untreated patients with HCC from the Italian Liver Cancer (ITA.LI.CA) database was performed. One-to-one matching according to demographic/etiologic/oncologic features was possible for 50 patients. The median OS for these 50 capecitabine-treated patients was 15.6 months, compared with a median OS of 8.0 months for the matched untreated patients (p = .043).
Conclusion: Metronomic capecitabine is well tolerated by patients with advanced HCC and appears to have activity both in treatment-naive patients and in those previously treated with sorafenib.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures

Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

