Establishment of a novel lingual organoid culture system: generation of organoids having mature keratinized epithelium from adult epithelial stem cells
- PMID: 24232854
- PMCID: PMC3828633
- DOI: 10.1038/srep03224
Establishment of a novel lingual organoid culture system: generation of organoids having mature keratinized epithelium from adult epithelial stem cells
Abstract
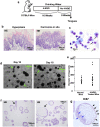
Despite the strong need for the establishment of a lingual epithelial cell culture system, a simple and convenient culture method has not yet been established. Here, we report the establishment of a novel lingual epithelium organoid culture system using a three-dimensional matrix and growth factors. Histological analyses showed that the generated organoids had both a stratified squamous epithelial cell layer and a stratum corneum. Very recently, we showed via a multicolor lineage tracing method that Bmi1-positive stem cells exist at the base of the epithelial basal layer in the interpapillary pit. Using our new culture system, we found that organoids could be generated by single Bmi1-positive stem cells and that in the established organoids, multiple Bmi1-positive stem cells were generated at the outermost layer. Moreover, we observed that organoids harvested at an early point in culture could be engrafted and maturate in the tongue of recipient mice and that the organoids generated from carcinogen-treated mice had an abnormal morphology. Thus, this culture system presents valuable settings for studying not only the regulatory mechanisms of lingual epithelium but also lingual regeneration and carcinogenesis.
Figures





Similar articles
-
Organoid Culture of Lingual Epithelial Cells in a Three-Dimensional Matrix.Methods Mol Biol. 2019;1576:93-99. doi: 10.1007/7651_2016_3. Methods Mol Biol. 2019. PMID: 27539458
-
Lingual Epithelial Stem Cells and Organoid Culture of Them.Int J Mol Sci. 2016 Jan 28;17(2):168. doi: 10.3390/ijms17020168. Int J Mol Sci. 2016. PMID: 26828484 Free PMC article. Review.
-
Identification of stem cells that maintain and regenerate lingual keratinized epithelial cells.Nat Cell Biol. 2013 May;15(5):511-8. doi: 10.1038/ncb2719. Epub 2013 Apr 7. Nat Cell Biol. 2013. PMID: 23563490
-
Bmi1-positive cells in the lingual epithelium could serve as cancer stem cells in tongue cancer.Sci Rep. 2016 Dec 22;6:39386. doi: 10.1038/srep39386. Sci Rep. 2016. PMID: 28004815 Free PMC article.
-
Advance in Human Epithelial-Derived Organoids Research.Mol Pharm. 2021 Nov 1;18(11):3931-3950. doi: 10.1021/acs.molpharmaceut.1c00452. Epub 2021 Sep 28. Mol Pharm. 2021. PMID: 34582198 Review.
Cited by
-
Characterization of normal and cancer stem-like cell populations in murine lingual epithelial organoids using single-cell RNA sequencing.Sci Rep. 2021 Nov 16;11(1):22329. doi: 10.1038/s41598-021-01783-5. Sci Rep. 2021. PMID: 34785704 Free PMC article.
-
Kidney organoids in translational medicine: Disease modeling and regenerative medicine.Dev Dyn. 2020 Jan;249(1):34-45. doi: 10.1002/dvdy.22. Epub 2019 Mar 26. Dev Dyn. 2020. PMID: 30843293 Free PMC article. Review.
-
Organoids in Tissue Transplantation.Adv Exp Med Biol. 2021;1347:45-64. doi: 10.1007/5584_2021_647. Adv Exp Med Biol. 2021. PMID: 34164796 Review.
-
Organoid Culture of Lingual Epithelial Cells in a Three-Dimensional Matrix.Methods Mol Biol. 2019;1576:93-99. doi: 10.1007/7651_2016_3. Methods Mol Biol. 2019. PMID: 27539458
-
Spatial transcriptomics reveals molecular cues underlying the site specificity of the adult mouse oral mucosa and its stem cell niches.Stem Cell Reports. 2024 Dec 10;19(12):1706-1719. doi: 10.1016/j.stemcr.2024.10.007. Epub 2024 Nov 27. Stem Cell Reports. 2024. PMID: 39547226 Free PMC article.
References
-
- Mbiene J.-P., Maccallum D. K. & Mistretta C. M. Organ cultures of embryonic rat tongue support tongue and gustatory papilla morphogenesis in vitro without intact sensory ganglia. J Comp Neurol 377, 324–340 (1997). - PubMed
-
- Zhou Y., Liu H.-X. & Mistretta C. M. Bone morphogenic proteins and noggin: Inhibiting and inducing fungiform taste papilla development. Develop Biol 297, 198–213 (2006). - PubMed
-
- Ookura T. et al. Fibroblast and epidermal growth factors modulate proliferation and neural cell adhesion molecule expression in epithelial cells derived from the adult mouse tongue. In Vitro Cell Dev Biol Anim 38, 365–372 (2002). - PubMed
-
- Luo X., Okubo T., Randell S. & Hogan B. L. M. Culture of endodermal stem/progenitor cells of the mouse tongue. In Vitro Cell Dev Biol Anim 45, 44–54 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

