The iron cycle in chronic kidney disease (CKD): from genetics and experimental models to CKD patients
- PMID: 24235084
- PMCID: PMC3910345
- DOI: 10.1093/ndt/gft443
The iron cycle in chronic kidney disease (CKD): from genetics and experimental models to CKD patients
Abstract
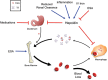
Iron is essential for most living organisms but iron excess can be toxic. Cellular and systemic iron balance is therefore tightly controlled. Iron homeostasis is dysregulated in chronic kidney disease (CKD) and contributes to the anemia that is prevalent in this patient population. Iron supplementation is one cornerstone of anemia management in CKD patients, but has not been rigorously studied in large prospective randomized controlled trials. This review highlights important advances from genetic studies and animal models that have provided key insights into the molecular mechanisms governing iron homeostasis and its disturbance in CKD, and summarizes how these findings may yield advances in the care of this patient population.
Keywords: anemia; chronic kidney disease; hepcidin; iron; review.
Figures



References
-
- Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. - PubMed
-
- McKie AT, Barrow D, Latunde-Dada GO, et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science. 2001;291:1755–1759. - PubMed
-
- Fleming MD, Trenor CC, III, Su MA, et al. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16:383–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

