Nuclear recruitment of neuronal nitric-oxide synthase by α-syntrophin is crucial for the induction of mitochondrial biogenesis
- PMID: 24235139
- PMCID: PMC3879559
- DOI: 10.1074/jbc.M113.506733
Nuclear recruitment of neuronal nitric-oxide synthase by α-syntrophin is crucial for the induction of mitochondrial biogenesis
Abstract
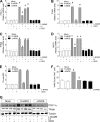
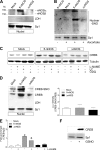
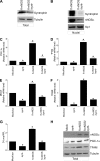
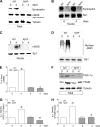
Neuronal nitric-oxide synthase (nNOS) has various splicing variants and different subcellular localizations. nNOS can be found also in the nucleus; however, its exact role in this compartment is still not completely defined. In this report, we demonstrate that the PDZ domain allows the recruitment of nNOS to nuclei, thus favoring local NO production, nuclear protein S-nitrosylation, and induction of mitochondrial biogenesis. In particular, overexpression of PDZ-containing nNOS (nNOSα) increases S-nitrosylated CREB with consequent augmented binding on cAMP response element consensus sequence on peroxisome proliferator-activated receptor γ co-activator (PGC)-1α promoter. The resulting PGC-1α induction is accompanied by the expression of mitochondrial genes (e.g., TFAM, MtCO1) and increased mitochondrial mass. Importantly, full active nNOS lacking PDZ domain (nNOSβ) does not localize in nuclei and fails in inducing the expression of PGC-1α. Moreover, we substantiate that the mitochondrial biogenesis normally accompanying myogenesis is associated with nuclear translocation of nNOS. We demonstrate that α-Syntrophin, which resides in nuclei of myocytes, functions as the upstream mediator of nuclear nNOS translocation and nNOS-dependent mitochondrial biogenesis. Overall, our results indicate that altered nNOS splicing and nuclear localization could be contributing factors in human muscular diseases associated with mitochondrial impairment.
Keywords: Mitochondria; Myogenesis; Nitric-oxide Synthase; S-Nitrosylation; Scaffold Proteins.
Figures





References
-
- Rotilio G., Aquilano K., Ciriolo M. R. (2003) Interplay of Cu,Zn superoxide dismutase and nitric oxide synthase in neurodegenerative processes. IUBMB Life 55, 629–634 - PubMed
-
- Zhou L., Zhu D. Y. (2009) Neuronal nitric oxide synthase. Structure, subcellular localization, regulation, and clinical implications. Nitric Oxide 20, 223–230 - PubMed
-
- Sullivan J. C., Pollock J. S. (2003) NOS 3 subcellular localization in the regulation of nitric oxide production. Acta Physiol. Scand. 179, 115–122 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

