Tubulation of endosomal structures in human dendritic cells by Toll-like receptor ligation and lymphocyte contact accompanies antigen cross-presentation
- PMID: 24235148
- PMCID: PMC3879573
- DOI: 10.1074/jbc.M113.511147
Tubulation of endosomal structures in human dendritic cells by Toll-like receptor ligation and lymphocyte contact accompanies antigen cross-presentation
Abstract
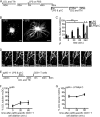
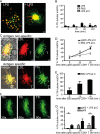
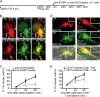
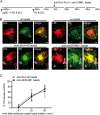
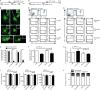
Mouse dendritic cells (DCs) can rapidly extend their Class II MHC-positive late endosomal compartments into tubular structures, induced by Toll-like receptor (TLR) triggering. Within antigen-presenting DCs, tubular endosomes polarize toward antigen-specific CD4(+) T cells, which are considered beneficial for their activation. Here we describe that also in human DCs, TLR triggering induces tubular late endosomes, labeled by fluorescent LDL. TLR triggering was insufficient for induced tubulation of transferrin-positive endosomal recycling compartments (ERCs) in human monocyte-derived DCs. We studied endosomal remodeling in human DCs in co-cultures of DCs with CD8(+) T cells. Tubulation of ERCs within human DCs requires antigen-specific CD8(+) T cell interaction. Tubular remodeling of endosomes occurs within 30 min of T cell contact and involves ligation of HLA-A2 and ICAM-1 by T cell-expressed T cell receptor and LFA-1, respectively. Disintegration of microtubules or inhibition of endosomal recycling abolished tubular ERCs, which coincided with reduced antigen-dependent CD8(+) T cell activation. Based on these data, we propose that remodeling of transferrin-positive ERCs in human DCs involves both innate and T cell-derived signals.
Keywords: Cell-Cell Interaction; Cross-presentation; Dendritic Cells; Endosomes; Intracellular Trafficking; Live Cell Confocal Microscopy; Recycling; T Cell Receptor; Toll-like Receptor (TLR); Tubulation.
Figures
References
-
- Villadangos J. A., Schnorrer P. (2007) Intrinsic and cooperative antigen-presenting functions of dendritic cell subsets in vivo. Nat. Rev. Immunol. 7, 543–555 - PubMed
-
- Burgdorf S., Schölz C., Kautz A., Tampé R., Kurts C. (2008) Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat. Immunol. 9, 558–566 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

