Survivin-induced abnormal ploidy contributes to cystic kidney and aneurysm formation
- PMID: 24235270
- PMCID: PMC3946000
- DOI: 10.1161/CIRCULATIONAHA.113.005746
Survivin-induced abnormal ploidy contributes to cystic kidney and aneurysm formation
Abstract
Background: Cystic kidneys and vascular aneurysms are clinical manifestations seen in patients with polycystic kidney disease, a cilia-associated pathology (ciliopathy). Survivin overexpression is associated with cancer, but the clinical pathology associated with survivin downregulation or knockout has never been studied before. The present studies aim to examine whether and how cilia function (Pkd1 or Pkd2) and structure (Tg737) play a role in cystic kidney and aneurysm through survivin downregulation.
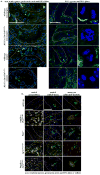
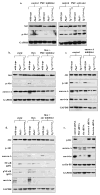
Methods and results: Cysts and aneurysms from polycystic kidney disease patients, Pkd mouse, and zebrafish models are characterized by chromosome instability and low survivin expression. This triggers cytokinesis defects and formation of nuclear polyploidy or aneuploidy. In vivo conditional mouse and zebrafish models confirm that survivin gene deletion in the kidneys results in a cystic phenotype. As in hypertensive Pkd1, Pkd2, and Tg737 models, aneurysm formation can also be induced in vascular-specific normotensive survivin mice. Survivin knockout also contributes to abnormal oriented cell division in both kidney and vasculature. Furthermore, survivin expression and ciliary localization are regulated by flow-induced cilia activation through protein kinase C, Akt and nuclear factor-κB. Circumventing ciliary function by re-expressing survivin can rescue polycystic kidney disease phenotypes.
Conclusions: For the first time, our studies offer a unifying mechanism that explains both renal and vascular phenotypes in polycystic kidney disease. Although primary cilia dysfunction accounts for aneurysm formation and hypertension, hypertension itself does not cause aneurysm. Furthermore, aneurysm formation and cyst formation share a common cellular and molecular pathway involving cilia function or structure, survivin expression, cytokinesis, cell ploidy, symmetrical cell division, and tissue architecture orientation.
Keywords: aurora kinase; blood flow; blood pressure; cardiovascular system; endothelium, vascular; epithelium.
Conflict of interest statement
Figures






References
-
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–37. - PubMed
-
- Xu C, Shmukler BE, Nishimura K, Kaczmarek E, Rossetti S, Harris PC, Wandinger-Ness A, Bacallao RL, Alper SL. Attenuated, flow-induced ATP release contributes to absence of flow-sensitive, purinergic Cai2+ signaling in human ADPKD cyst epithelial cells. Am J Physiol Renal Physiol. 2009;296:F1464–76. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

