Characterization and kinetic properties of the purified Trematosphaeria mangrovei laccase enzyme
- PMID: 24235874
- PMCID: PMC3824134
- DOI: 10.1016/j.sjbs.2013.04.001
Characterization and kinetic properties of the purified Trematosphaeria mangrovei laccase enzyme
Abstract
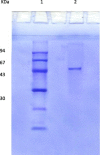
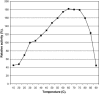
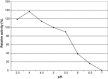
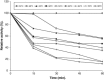
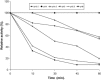
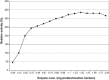
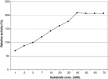
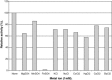
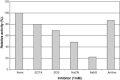
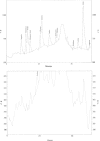
The properties of Trematosphaeria mangrovei laccase enzyme purified on Sephadex G-100 column were investigated. SDS-PAGE of the purified laccase enzyme showed a single band at 48 kDa. The pure laccase reached its maximal activity at temperature 65 °C, pH 4.0 with K m equal 1.4 mM and V max equal 184.84 U/mg protein. The substrate specificity of the purified laccase was greatly influenced by the nature and position of the substituted groups in the phenolic ring. The pure laccase was tested with some metal ions and inhibitors, FeSO4 completely inhibited laccase enzyme and also highly affected by (NaN3) at a concentration of 1 mM. Amino acid composition of the pure enzyme was also determined. Carbohydrate content of purified laccase enzyme was 23% of the enzyme sample. The UV absorption spectra of the purified laccase enzyme showed a single peak at 260-280 nm.
Keywords: Characterization; Laccase; Marine-derived fungi; Trematosphaeria mangrovei.
Figures


References
-
- Atalla M.M., Zeinab H.K., Eman R.H., Amani A.Y., Abeer A.A. Screening of some marine-derived fungal isolates for lignin degrading enzymes (LDEs) production. Agric. Biol. J. North America. 2010;1:591–599.
-
- Baldrian P. Purification and characterization of laccase from the white-rot fungus Daedalea quercina and decolorization of synthetic dyes by the enzyme. Appl. Microbiol. Biotechnol. 2004;63:560–563. - PubMed
-
- Baldrian P. Fungal laccases-occurrence and properties. FEMS Microbiol. Rev. 2006;30:215–242. - PubMed
-
- Banerjee U.C., Vohra R.M. Production of laccase by Curvularia sp. Folia Microbial. 1991;36:343. - PubMed
-
- Ben Younes S., Mechichi T., Sayadi S. Purification and characterization of the laccase secreted by the white rot fungus Perenniporia tephropora and its role in the decolourization of synthetic dyes. J Appl. Microbiol. 2007;102:1033–1042. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

