Electron Ionization-Induced Release of Coded Isotopic Reporter Ions in an m/z Zone of Minimal Interference for Quantifiable, Multiplexed GC-MS Analyses
- PMID: 24235976
- PMCID: PMC3822575
- DOI: 10.1039/C3AY41124A
Electron Ionization-Induced Release of Coded Isotopic Reporter Ions in an m/z Zone of Minimal Interference for Quantifiable, Multiplexed GC-MS Analyses
Abstract
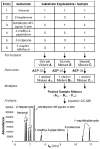
We describe an isotope coding strategy that enables simultaneous GC-MS analysis of multiple samples for substrate identification and quantification. The method relies on direct measurement of isotopic ethyl carbenium ions serving as mass spectral tags in a zone of minimal interference (ZMI) at m/z 31-37. Sample aldehyde and ketone mixtures were reacted with isotopic 2-aminooxyethyl propionates to illustrate the method, which determined the relative abundance of the mixed compounds with an average 95% accuracy. ZMI reporter ion detection also enables chemoselective substrate profiling and absolute quantification, as demonstrated using a biologically derived sample.
Figures







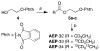
References
-
- Almstetter MF, Oefner PJ, Dettmer K. Anal Bioanal Chem. 2012;402:1993–2013. - PubMed
-
- Wei R, Li G, Seymour AB. Anal Chem. 2010;82:5527–5533. - PubMed
-
- Courant F, Pinel G, Bichon E, Monteau F, Antignac JP, Le Bizec B. Analyst. 2009;134:1637–1646. - PubMed
- Lv H, Palacios G, Hartil K, Kurland IJ. J Proteome Res. 2011;10:2104–2112. - PMC - PubMed
- Aoki M, Konya Y, Takagaki T, Umemura K, Sogame Y, Katsumata T, Komuro S. Rapid Commun Mass Spectrom. 2011;25:1847–1852. - PubMed
- Spagou K, Wilson ID, Masson P, Theodoridis G, Raikos N, Coen M, Holmes E, Lindon JC, Plumb RS, Nicholson JK, Want EJ. Anal Chem. 2011;83:382–390. - PubMed
- Xiao JF, Zhou B, Ressom HW. Trends Anal Chem. 2012;32:1–14. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

