Lipopolysaccharide induces degradation of connexin43 in rat astrocytes via the ubiquitin-proteasome proteolytic pathway
- PMID: 24236122
- PMCID: PMC3827358
- DOI: 10.1371/journal.pone.0079350
Lipopolysaccharide induces degradation of connexin43 in rat astrocytes via the ubiquitin-proteasome proteolytic pathway
Abstract
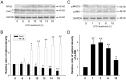
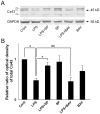
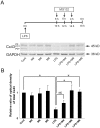
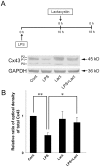
The astrocytic syncytium plays a critical role in maintaining the homeostasis of the brain through the regulation of gap junction intercellular communication (GJIC). Changes to GJIC in response to inflammatory stimuli in astrocytes may have serious effects on the brain. We have previously shown that lipopolysaccharide (LPS) reduces connexin43 (Cx43) expression and GJIC in cultured rat astrocytes via a toll-like receptor 4-mediated signaling pathway. In the present study, treatment of astrocytes with LPS resulted in a significant increase in levels of the phosphorylated forms of stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) -1, -2, and -3 for up to 18 h. An increase in nuclear transcription factor NF-κB levels was also observed after 8 h of LPS treatment and was sustained for up to 18 h. The LPS-induced decrease in Cx43 protein levels and inhibition of GJIC were blocked by the SAPK/JNK inhibitor SP600125, but not by the NF-κB inhibitor BAY11-7082. Following blockade of de novo protein synthesis by cycloheximide, LPS accelerated Cx43 degradation. Moreover, the LPS-induced downregulation of Cx43 was blocked following inhibition of 26S proteasome activity using the reversible proteasome inhibitor MG132 or the irreversible proteasome inhibitor lactacystin. Immunoprecipitation analyses revealed an increased association of Cx43 with both ubiquitin and E3 ubiquitin ligase Nedd4 in astrocytes after LPS stimulation for 6 h and this effect was prevented by SP600125. Taken together, these results suggest that LPS stimulation leads to downregulation of Cx43 expression and GJIC in rat astrocytes by activation of SAPK/JNK and the ubiquitin-proteasome proteolytic pathway.
Conflict of interest statement
Figures




Similar articles
-
Lipopolysaccharide-induced inhibition of connexin43 gap junction communication in astrocytes is mediated by downregulation of caveolin-3.Int J Biochem Cell Biol. 2010 May;42(5):762-70. doi: 10.1016/j.biocel.2010.01.016. Epub 2010 Jan 20. Int J Biochem Cell Biol. 2010. PMID: 20093193
-
Proinflammatory cytokines downregulate connexin 43-gap junctions via the ubiquitin-proteasome system in rat spinal astrocytes.Biochem Biophys Res Commun. 2015 Sep 4;464(4):1202-1208. doi: 10.1016/j.bbrc.2015.07.105. Epub 2015 Jul 23. Biochem Biophys Res Commun. 2015. PMID: 26212436
-
Antofine-induced connexin43 gap junction disassembly in rat astrocytes involves protein kinase Cβ.Neurotoxicology. 2013 Mar;35:169-79. doi: 10.1016/j.neuro.2013.02.001. Epub 2013 Feb 10. Neurotoxicology. 2013. PMID: 23403203
-
Ubiquitination of gap junction proteins.J Membr Biol. 2007 Jun;217(1-3):43-51. doi: 10.1007/s00232-007-9050-z. Epub 2007 Jul 28. J Membr Biol. 2007. PMID: 17657522 Review.
-
Regulation of connexins by the ubiquitin system: Implications for intercellular communication and cancer.Biochim Biophys Acta. 2016 Apr;1865(2):133-46. doi: 10.1016/j.bbcan.2016.02.001. Epub 2016 Feb 12. Biochim Biophys Acta. 2016. PMID: 26855059 Review.
Cited by
-
Ginsenoside Rg1 Ameliorates Neuroinflammation via Suppression of Connexin43 Ubiquitination to Attenuate Depression.Front Pharmacol. 2021 Aug 5;12:709019. doi: 10.3389/fphar.2021.709019. eCollection 2021. Front Pharmacol. 2021. PMID: 34421601 Free PMC article.
-
Systemic LPS Administration Stimulates the Activation of Non-Neuronal Cells in an Experimental Model of Spinal Muscular Atrophy.Cells. 2024 May 4;13(9):785. doi: 10.3390/cells13090785. Cells. 2024. PMID: 38727321 Free PMC article.
-
Astroglia in Sepsis Associated Encephalopathy.Neurochem Res. 2020 Jan;45(1):83-99. doi: 10.1007/s11064-019-02743-2. Epub 2019 Feb 18. Neurochem Res. 2020. PMID: 30778837 Free PMC article. Review.
-
Astrocyte Networks as Therapeutic Targets in Glaucomatous Neurodegeneration.Cells. 2021 Jun 2;10(6):1368. doi: 10.3390/cells10061368. Cells. 2021. PMID: 34199470 Free PMC article. Review.
-
The Double-Edged Effect of Connexins and Pannexins of Glial Cells in Central and Peripheral Nervous System After Nerve Injury.Mol Neurobiol. 2025 May 1. doi: 10.1007/s12035-025-04991-6. Online ahead of print. Mol Neurobiol. 2025. PMID: 40310549 Review.
References
-
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N (2010) Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci 11: 87–99. - PubMed
-
- Giaume C, Theis M (2010) Pharmacological and genetic approaches to study connexin-mediated channels in glial cells of the central nervous system. Brain Res Rev 63: 160–176. - PubMed
-
- Dermietzel R, Gao Y, Scemes E, Vieira D, Urban M, et al. (2000) Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res Brain Res Rev 32: 45–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

