A simple alkaline method for decellularizing human amniotic membrane for cell culture
- PMID: 24236148
- PMCID: PMC3827346
- DOI: 10.1371/journal.pone.0079632
A simple alkaline method for decellularizing human amniotic membrane for cell culture
Abstract
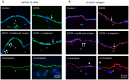
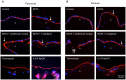
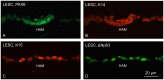
Human amniotic membrane is a standard substratum used to culture limbal epithelial stem cells for transplantation to patients with limbal stem cell deficiency. Various methods were developed to decellularize amniotic membrane, because denuded membrane is poorly immunogenic and better supports repopulation by dissociated limbal epithelial cells. Amniotic membrane denuding usually involves treatment with EDTA and/or proteolytic enzymes; in many cases additional mechanical scraping is required. Although ensuring limbal cell proliferation, these methods are not standardized, require relatively long treatment times and can result in membrane damage. We propose to use 0.5 M NaOH to reliably remove amniotic cells from the membrane. This method was used before to lyse cells for DNA isolation and radioactivity counting. Gently rubbing a cotton swab soaked in NaOH over the epithelial side of amniotic membrane leads to nearly complete and easy removal of adherent cells in less than a minute. The denuded membrane is subsequently washed in a neutral buffer. Cell removal was more thorough and uniform than with EDTA, or EDTA plus mechanical scraping with an electric toothbrush, or n-heptanol plus EDTA treatment. NaOH-denuded amniotic membrane did not show any perforations compared with mechanical or thermolysin denuding, and showed excellent preservation of immunoreactivity for major basement membrane components including laminin α2, γ1-γ3 chains, α1/α2 and α6 type IV collagen chains, fibronectin, nidogen-2, and perlecan. Sodium hydroxide treatment was efficient with fresh or cryopreserved (10% dimethyl sulfoxide or 50% glycerol) amniotic membrane. The latter method is a common way of membrane storage for subsequent grafting in the European Union. NaOH-denuded amniotic membrane supported growth of human limbal epithelial cells, immortalized corneal epithelial cells, and induced pluripotent stem cells. This simple, fast and reliable method can be used to standardize decellularized amniotic membrane preparations for expansion of limbal stem cells in vitro before transplantation to patients.
Conflict of interest statement
Figures





Similar articles
-
Comparative analysis of the basement membrane composition of the human limbus epithelium and amniotic membrane epithelium.Cornea. 2012 May;31(5):564-9. doi: 10.1097/ICO.0b013e3182254b78. Cornea. 2012. PMID: 22382594
-
Cultured corneal epithelia for ocular surface disease.Trans Am Ophthalmol Soc. 1999;97:891-986. Trans Am Ophthalmol Soc. 1999. PMID: 10703147 Free PMC article.
-
Comparison of intact and denuded amniotic membrane as a substrate for cell-suspension culture of human limbal epithelial cells.Graefes Arch Clin Exp Ophthalmol. 2007 Jan;245(1):123-34. doi: 10.1007/s00417-005-0095-3. Epub 2006 Apr 13. Graefes Arch Clin Exp Ophthalmol. 2007. PMID: 16612639
-
Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche.Surv Ophthalmol. 2003 Nov-Dec;48(6):631-46. doi: 10.1016/j.survophthal.2003.08.003. Surv Ophthalmol. 2003. PMID: 14609709 Review.
-
Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting-a review.Cell Tissue Bank. 2017 Jun;18(2):193-204. doi: 10.1007/s10561-017-9618-5. Epub 2017 Mar 2. Cell Tissue Bank. 2017. PMID: 28255771 Review.
Cited by
-
Gamma-irradiated human amniotic membrane decellularised with sodium dodecyl sulfate is a more efficient substrate for the ex vivo expansion of limbal stem cells.Acta Biomater. 2017 Oct 1;61:124-133. doi: 10.1016/j.actbio.2017.07.041. Epub 2017 Jul 29. Acta Biomater. 2017. PMID: 28760619 Free PMC article.
-
Integrated Transcriptome and Proteome Analyses Reveal the Regulatory Role of miR-146a in Human Limbal Epithelium via Notch Signaling.Cells. 2020 Sep 26;9(10):2175. doi: 10.3390/cells9102175. Cells. 2020. PMID: 32993109 Free PMC article.
-
Development of a tissue-engineered skin substitute on a base of human amniotic membrane.J Tissue Eng. 2019 Feb 2;10:2041731418825378. doi: 10.1177/2041731418825378. eCollection 2019 Jan-Dec. J Tissue Eng. 2019. PMID: 30746119 Free PMC article.
-
Non-mulberry Silk Fibroin Biomaterial for Corneal Regeneration.Sci Rep. 2016 Feb 24;6:21840. doi: 10.1038/srep21840. Sci Rep. 2016. PMID: 26908015 Free PMC article.
-
Human amniotic membrane scaffold enhances adipose mesenchymal stromal cell mitochondrial bioenergetics promoting their regenerative capacities.Mol Cell Biochem. 2025 Apr;480(4):2611-2632. doi: 10.1007/s11010-024-05094-x. Epub 2024 Oct 25. Mol Cell Biochem. 2025. PMID: 39453499
References
-
- Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, et al. (2010) Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med 363: 147–155. - PubMed
-
- Ahmad S, Kolli S, Lako M, Figueiredo F, Daniels JT (2010) Stem cell therapies for ocular surface disease. Drug Discov Today 15: 306–313. - PubMed
-
- Ordonez P, Di Girolamo N (2012) Limbal epithelial stem cells: role of the niche microenvironment. Stem Cells 30: 100–107. - PubMed
-
- Biber JM, Holland EJ, Neff KD (2010) Management of ocular stem cell disease. Int Ophthalmol Clin 50: 25–34. - PubMed
-
- Mason SL, Stewart RM, Kearns VR, Williams RL, Sheridan CM (2011) Ocular epithelial transplantation: current uses and future potential. Regen Med 6: 767–782. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

