Spo0A differentially regulates toxin production in evolutionarily diverse strains of Clostridium difficile
- PMID: 24236153
- PMCID: PMC3827441
- DOI: 10.1371/journal.pone.0079666
Spo0A differentially regulates toxin production in evolutionarily diverse strains of Clostridium difficile
Abstract
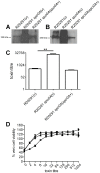
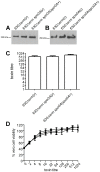
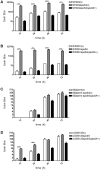
Clostridium difficile is an important pathogen of humans and animals, representing a significant global healthcare problem. The last decade has seen the emergence of epidemic BI/NAP1/027 and ribotype 078 isolates, associated with the onset of more severe disease and higher rates of morbidity and mortality. However, little is known about these isolates at the molecular level, partly due to difficulties in the genetic manipulation of these strains. Here we report the development of an optimised Tn916-mediated plasmid transfer system, and the use of this system to construct and complement spo0A mutants in a number of different C. difficile strain backgrounds. Spo0A is a global regulator known to control sporulation, but may also be involved in the regulation of potential virulence factors and other phenotypes. Recent studies have failed to elucidate the role of Spo0A in toxin A and toxin B production by C. difficile, with conflicting data published to date. In this study, we aimed to clarify the role of Spo0A in production of the major toxins by C. difficile. Through the construction and complementation of spo0A mutants in two ribotype 027 isolates, we demonstrate that Spo0A acts as a negative regulator of toxin A and toxin B production in this strain background. In addition, spo0A was disrupted and subsequently complemented in strain 630Δerm and, for the first time, in a ribotype 078 isolate, JGS6133. In contrast to the ribotype 027 strains, Spo0A does not appear to regulate toxin production in strain 630Δerm. In strain JGS6133, Spo0A appears to negatively regulate toxin production during early stationary phase, but has little effect on toxin expression during late stationary phase. These data suggest that Spo0A may differentially regulate toxin production in phylogenetically distinct C. difficile strain types. In addition, Spo0A may be involved in regulating some aspects of C. difficile motility.
Conflict of interest statement
Figures






Similar articles
-
Characterization of Flagellum and Toxin Phase Variation in Clostridioides difficile Ribotype 012 Isolates.J Bacteriol. 2018 Jun 25;200(14):e00056-18. doi: 10.1128/JB.00056-18. Print 2018 Jul 15. J Bacteriol. 2018. PMID: 29735765 Free PMC article.
-
C. difficile 630Δerm Spo0A regulates sporulation, but does not contribute to toxin production, by direct high-affinity binding to target DNA.PLoS One. 2012;7(10):e48608. doi: 10.1371/journal.pone.0048608. Epub 2012 Oct 31. PLoS One. 2012. PMID: 23119071 Free PMC article.
-
Characterization of the sporulation initiation pathway of Clostridium difficile and its role in toxin production.J Bacteriol. 2009 Dec;191(23):7296-305. doi: 10.1128/JB.00882-09. Epub 2009 Sep 25. J Bacteriol. 2009. PMID: 19783633 Free PMC article.
-
Regulatory networks: Linking toxin production and sporulation in Clostridioides difficile.Anaerobe. 2025 Feb;91:102920. doi: 10.1016/j.anaerobe.2024.102920. Epub 2024 Nov 7. Anaerobe. 2025. PMID: 39521117 Review.
-
The role of flagella in Clostridium difficile pathogenicity.Trends Microbiol. 2015 May;23(5):275-82. doi: 10.1016/j.tim.2015.01.004. Epub 2015 Feb 4. Trends Microbiol. 2015. PMID: 25659185 Review.
Cited by
-
Clostridium difficile infection.Nat Rev Dis Primers. 2016 Apr 7;2:16020. doi: 10.1038/nrdp.2016.20. Nat Rev Dis Primers. 2016. PMID: 27158839 Free PMC article. Review.
-
Spores of Clostridioides difficile are toxin delivery vehicles.Commun Biol. 2024 Jul 10;7(1):839. doi: 10.1038/s42003-024-06521-x. Commun Biol. 2024. PMID: 38987278 Free PMC article.
-
Sporulation and Germination in Clostridial Pathogens.Microbiol Spectr. 2019 Nov;7(6):10.1128/microbiolspec.gpp3-0017-2018. doi: 10.1128/microbiolspec.GPP3-0017-2018. Microbiol Spectr. 2019. PMID: 31858953 Free PMC article. Review.
-
The HtrA-like protease CD3284 modulates virulence of Clostridium difficile.Infect Immun. 2014 Oct;82(10):4222-32. doi: 10.1128/IAI.02336-14. Epub 2014 Jul 21. Infect Immun. 2014. PMID: 25047848 Free PMC article.
-
Emergence of a non-sporulating secondary phenotype in Clostridium (Clostridioides) difficile ribotype 078 isolated from humans and animals.Sci Rep. 2019 Sep 23;9(1):13722. doi: 10.1038/s41598-019-50285-y. Sci Rep. 2019. PMID: 31548637 Free PMC article.
References
-
- Borriello SP (1998) Pathogenesis of Clostridium difficile infection. J Antimicrob Chemother 41: 13–19. - PubMed
-
- Keel MK, Songer JG (2006) The comparative pathology of Clostridium difficile-associated disease. Vet Pathol 43: 225–240. - PubMed
-
- Keessen EC, Gaastra W, Lipman LJ (2011) Clostridium difficile infection in humans and animals, differences and similarities. Vet Microbiol 153: 205–217. - PubMed
-
- Aktories K (2011) Bacterial protein toxins that modify host regulatory GTPases. Nat Rev Microbiol 9: 487–498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

