Characterization of the vaginal microbiota among sexual risk behavior groups of women with bacterial vaginosis
- PMID: 24236175
- PMCID: PMC3827412
- DOI: 10.1371/journal.pone.0080254
Characterization of the vaginal microbiota among sexual risk behavior groups of women with bacterial vaginosis
Erratum in
- PLoS One. 2013;8(12). doi:10.1371/annotation/f7674ab1-fbd5-4293-ad2c-d1a795962e8b. Swiatlo, Edwin [added]
Abstract
Background: The pathogenesis of bacterial vaginosis (BV) remains elusive. BV may be more common among women who have sex with women (WSW). The objective of this study was to use 454 pyrosequencing to investigate the vaginal microbiome of WSW, women who have sex with women and men (WSWM), and women who have sex with men (WSM) with BV to determine if there are differences in organism composition between groups that may inform new hypotheses regarding the pathogenesis of BV.
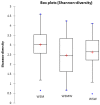
Methods: Vaginal swab specimens from eligible women with BV at the Mississippi State Department of Health STD Clinic were used. After DNA extraction, 454 pyrosequencing of PCR-amplified 16S rRNA gene sequences was performed. Sequence data was classified using the Ribosomal Database Program classifer. Complete linkage clustering analysis was performed to compare bacterial community composition among samples. Differences in operational taxonomic units with an abundance of ≥ 2% between risk behavior groups were determined. Alpha and beta diversity were measured using Shannon's Index implemented in QIIME and Unifrac analysis, respectively.
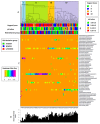
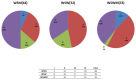
Results: 33 WSW, 35 WSWM, and 44 WSM were included. The vaginal bacterial communities of all women clustered into four taxonomic groups with the dominant taxonomic group in each being Lactobacillus, Lachnospiraceae, Prevotella, and Sneathia. Regarding differences in organism composition between risk behavior groups, the abundance of Atopobium (relative ratio (RR)=0.24; 95%CI 0.11-0.54) and Parvimonas (RR=0.33; 95%CI 0.11-0.93) were significantly lower in WSW than WSM, the abundance of Prevotella was significantly higher in WSW than WSWM (RR=1.77; 95%CI 1.10-2.86), and the abundance of Atopobium (RR=0.41; 95%CI 0.18-0.88) was significantly lower in WSWM than WSM. Overall, WSM had the highest diversity of bacterial taxa.
Conclusion: The microbiology of BV among women in different risk behavior groups is heterogeneous. WSM in this study had the highest diversity of bacterial taxa. Additional studies are needed to better understand these differences.
Conflict of interest statement
Figures



Similar articles
-
Comparisons of vaginal flora patterns among sexual behaviour groups of women: implications for the pathogenesis of bacterial vaginosis.Sex Health. 2018 Feb;15(1):61-67. doi: 10.1071/SH17087. Sex Health. 2018. PMID: 29212588 Free PMC article.
-
Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis.BMC Genomics. 2010 Sep 7;11:488. doi: 10.1186/1471-2164-11-488. BMC Genomics. 2010. PMID: 20819230 Free PMC article.
-
Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria.PLoS One. 2012;7(6):e37818. doi: 10.1371/journal.pone.0037818. Epub 2012 Jun 18. PLoS One. 2012. PMID: 22719852 Free PMC article.
-
Characterization of the vaginal microflora in health and disease.Dan Med J. 2014 Apr;61(4):B4830. Dan Med J. 2014. PMID: 24814599 Review.
-
Vaginal microbiome.Ceska Gynekol. 2018 Winter;83(5):371-379. Ceska Gynekol. 2018. PMID: 30848142 Review. English.
Cited by
-
Therapeutic Opportunities in the Vaginal Microbiome.Microbiol Spectr. 2017 Jun;5(3):10.1128/microbiolspec.bad-0001-2016. doi: 10.1128/microbiolspec.BAD-0001-2016. Microbiol Spectr. 2017. PMID: 28597813 Free PMC article. Review.
-
Association between BVAB1 and high Nugent scores among women with bacterial vaginosis.Diagn Microbiol Infect Dis. 2014 Dec;80(4):321-3. doi: 10.1016/j.diagmicrobio.2014.09.008. Epub 2014 Sep 16. Diagn Microbiol Infect Dis. 2014. PMID: 25262105 Free PMC article.
-
Factors Associated with Bacterial Vaginosis among Women Who Have Sex with Women: A Systematic Review.PLoS One. 2015 Dec 16;10(12):e0141905. doi: 10.1371/journal.pone.0141905. eCollection 2015. PLoS One. 2015. PMID: 26675816 Free PMC article.
-
In Silico and Experimental Evaluation of Primer Sets for Species-Level Resolution of the Vaginal Microbiota Using 16S Ribosomal RNA Gene Sequencing.J Infect Dis. 2019 Jan 7;219(2):305-314. doi: 10.1093/infdis/jiy508. J Infect Dis. 2019. PMID: 30535155 Free PMC article.
-
Effect of metronidazole on vaginal microbiota associated with asymptomatic bacterial vaginosis.Access Microbiol. 2021 May 4;3(5):000226. doi: 10.1099/acmi.0.000226. eCollection 2021. Access Microbiol. 2021. PMID: 34151180 Free PMC article.
References
-
- Rein MF, Holmes KK (1983) Non-specific vaginitis, vulvovaginal candidiasis, and trichomoniasis. In: Remington JS, Swartz MN. Current Clinical Topics in Infectious Diseases. New York: McGraw-Hill; pp. 281-315.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

