TGFβs modulate permeability of the blood-epididymis barrier in an in vitro model
- PMID: 24236189
- PMCID: PMC3827453
- DOI: 10.1371/journal.pone.0080611
TGFβs modulate permeability of the blood-epididymis barrier in an in vitro model
Abstract
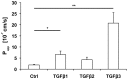
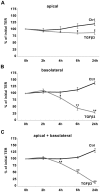
The blood-epididymis barrier (BEB) is formed by epithelial tight junctions mediating selective permeability of the epididymal epithelium. Defective barrier function can disturb the balance of the epididymal milieu, which may result in infertility. The stroma of the epididymis contains high amounts of cytokines of the TGFβ family of unknown function. We screened possible effects of all three TGFβ isoforms on paracellular tightness in a BEB in vitro model based on the strongly polarized mouse epididymal epithelial MEPC5 cells in the transwell system. In this model we found a robust transepithelial electrical resistance (TER) of about 840 Ω x cm(2). Effects on the paracellular permeability were evaluated by two methods, TER and FITC-Dextran-based tracer diffusion assays. Both assays add up to corresponding results indicating a time-dependent disturbance of the BEB differentially for the three TGFβ isoforms (TGFβ3>TGFβ1>TGFβ2) in a TGFβ-receptor-1 kinase- and Smad-dependent manner. The tight junction protein claudin-1 was found to be reduced by the treatment with TGFβs, whereas occludin was not influenced. Epididymal epithelial cells are predominantly responsive to TGFβs from the basolateral side, suggesting that TGFβ may have an impact on the epididymal epithelium from the stroma in vivo. Our data show for the first time that TGFβs decrease paracellular tightness in epididymal epithelial cells, thus establishing a novel mechanism of regulation of BEB permeability, which is elementary for sperm maturation and male fertility.
Conflict of interest statement
Figures





Similar articles
-
Colon epithelial cell TGFβ signaling modulates the expression of tight junction proteins and barrier function in mice.Am J Physiol Gastrointest Liver Physiol. 2021 Jun 1;320(6):G936-G957. doi: 10.1152/ajpgi.00053.2021. Epub 2021 Mar 24. Am J Physiol Gastrointest Liver Physiol. 2021. PMID: 33759564 Free PMC article.
-
Metformin prevents the effects of Pseudomonas aeruginosa on airway epithelial tight junctions and restricts hyperglycaemia-induced bacterial growth.J Cell Mol Med. 2016 Apr;20(4):758-64. doi: 10.1111/jcmm.12784. Epub 2016 Feb 2. J Cell Mol Med. 2016. PMID: 26837005 Free PMC article.
-
Protamine-induced epithelial barrier disruption involves rearrangement of cytoskeleton and decreased tight junction-associated protein expression in cultured MDCK strains.Cell Struct Funct. 2005 Feb;29(5-6):165-78. doi: 10.1247/csf.29.165. Cell Struct Funct. 2005. PMID: 15840948
-
The role of molecular remodeling in differential regulation of tight junction permeability.Semin Cell Dev Biol. 2014 Dec;36:204-12. doi: 10.1016/j.semcdb.2014.09.022. Epub 2014 Sep 28. Semin Cell Dev Biol. 2014. PMID: 25263012 Free PMC article. Review.
-
Epithelial dynamics in the epididymis: role in the maturation, protection, and storage of spermatozoa.Andrology. 2019 Sep;7(5):631-643. doi: 10.1111/andr.12632. Epub 2019 May 1. Andrology. 2019. PMID: 31044554 Free PMC article. Review.
Cited by
-
Open chromatin mapping identifies transcriptional networks regulating human epididymis epithelial function.Mol Hum Reprod. 2014 Dec;20(12):1198-207. doi: 10.1093/molehr/gau075. Epub 2014 Sep 1. Mol Hum Reprod. 2014. PMID: 25180270 Free PMC article.
-
Oridonin Preserves Retinal Pigmented Epithelial Cell Tight Junctions and Ameliorates Choroidal Neovascularization.Invest Ophthalmol Vis Sci. 2025 Feb 3;66(2):56. doi: 10.1167/iovs.66.2.56. Invest Ophthalmol Vis Sci. 2025. PMID: 39982392 Free PMC article.
-
The epididymal immune balance: a key to preserving male fertility.Asian J Androl. 2019 Nov-Dec;21(6):531-539. doi: 10.4103/aja.aja_11_19. Asian J Androl. 2019. PMID: 30924450 Free PMC article. Review.
-
Molecular Pathways Implicated in the Differentiation and Function of Epididymal Basal Cells.Adv Exp Med Biol. 2025;1469:89-113. doi: 10.1007/978-3-031-82990-1_5. Adv Exp Med Biol. 2025. PMID: 40301254 Review.
-
Zika virus disrupts the barrier structure and Absorption/Secretion functions of the epididymis in mice.PLoS Negl Trop Dis. 2021 Mar 5;15(3):e0009211. doi: 10.1371/journal.pntd.0009211. eCollection 2021 Mar. PLoS Negl Trop Dis. 2021. PMID: 33667230 Free PMC article.
References
-
- Hinton BT, Galdamez MM, Sutherland A, Bomgardner D, Xu B, et al. (2011) How do you get six meters of epididymis inside a human scrotum? J Androl 32: 558–564. - PubMed
-
- Gregory M, Cyr DG (2006) Identification of multiple claudins in the rat epididymis. Mol Reprod Dev 73: 580–588. - PubMed
-
- Cyr DG, Gregory M, Dube E, Dufresne J, Chan PTK, et al. (2007) Orchestration of occludins, claudins, catenins and cadherins as players involved in maintenance of the blood-epididymal barrier in animals and humans. Asian J Andrology 9: 463–475. - PubMed
-
- Dube E, Dufresne J, Chan PTK, Hermo L, Cyr DG (2010) Assessing the role of claudins in maintaining the integrity of epididymal tight junctions using novel human epididymal cell lines. Biol Reprod 82: 1119–1128. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

