Evaluation of NK cell function by flowcytometric measurement and impedance based assay using real-time cell electronic sensing system
- PMID: 24236291
- PMCID: PMC3819884
- DOI: 10.1155/2013/210726
Evaluation of NK cell function by flowcytometric measurement and impedance based assay using real-time cell electronic sensing system
Abstract
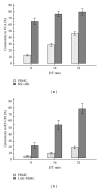
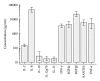
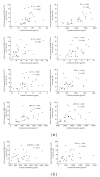
Although real-time cell electronic sensing (RT-CES) system-based natural killer (NK) cytotoxicity has been introduced, it has not been evaluated using human blood samples. In present study, we measured flowcytometry based assay (FCA) and RT-CES based NK cytotoxicity and analyzed degranulation activity (CD107a) and cytokine production. In 98 healthy individuals, FCA with peripheral blood mononuclear cells (PBMCs) at effector to target (E/T) ratio of 32 revealed 46.5 ± 2.6% cytolysis of K562 cells, and 23.5 ± 1.1% of NK cells showed increased degranulation. In RT-CES system, adherent NIH3T3 target cells were resistant to basal killing by PBMC or NK cells. NK cell activation by adding IL-2 demonstrated real-time dynamic killing activity, and lymphokine-activated PBMC (E/T ratio of 32) from 15 individuals showed 59.1 ± 6.2% cytotoxicity results after 4 hours incubation in RT-CES system. However, there was no significant correlation between FCA and RT-CES cytotoxicity. After K562 target cell stimulation, PBMC produced profound proinflammatory and immunoregulatory cytokines/chemokines including IL-2, IL-8, IL-10, MIP-1 α β , IFN- γ , and TNF- α , and cytokine/chemokine secretion was related to flowcytometry-based NK cytotoxicity. These data suggest that RT-CES and FCA differ in sensitivity, applicability and providing information, and further investigations are necessary in variable clinical conditions.
Figures



Similar articles
-
Measurement of NK activity in whole blood by the CD69 up-regulation after co-incubation with K562, comparison with NK cytotoxicity assays and CD107a degranulation assay.J Immunol Methods. 2011 Sep 30;372(1-2):187-95. doi: 10.1016/j.jim.2011.07.016. Epub 2011 Aug 2. J Immunol Methods. 2011. PMID: 21839083
-
Enhancement of NK Cell Cytotoxic Activity and Immunoregulatory Effects of a Natural Product Supplement Across a Wide Age Span: A 30-Day In Vivo Human Study.Int J Mol Sci. 2025 Mar 22;26(7):2897. doi: 10.3390/ijms26072897. Int J Mol Sci. 2025. PMID: 40243481 Free PMC article.
-
A Flow Cytometry-Based Whole Blood Natural Killer Cell Cytotoxicity Assay Using Overnight Cytokine Activation.Front Immunol. 2020 Aug 14;11:1851. doi: 10.3389/fimmu.2020.01851. eCollection 2020. Front Immunol. 2020. PMID: 32922399 Free PMC article.
-
An improved flow cytometry-based natural killer cytotoxicity assay involving calcein AM staining of effector cells.Ann Clin Lab Sci. 2012 Winter;42(1):42-9. Ann Clin Lab Sci. 2012. PMID: 22371909
-
Dual effects of cytokines in regulation of MHC-unrestricted cell mediated cytotoxicity.Crit Rev Immunol. 1993;13(1):1-34. Crit Rev Immunol. 1993. PMID: 8466640 Review.
Cited by
-
Droplet-Based Cytotoxicity Assay: Implementation of Time-Efficient Screening of Antitumor Activity of Natural Killer Cells.ACS Omega. 2020 Sep 17;5(38):24674-24683. doi: 10.1021/acsomega.0c03264. eCollection 2020 Sep 29. ACS Omega. 2020. PMID: 33015484 Free PMC article.
-
Application of an improved flow cytometry-based NK cell activity assay in adult hemophagocytic lymphohistiocytosis.Int J Hematol. 2017 Jun;105(6):828-834. doi: 10.1007/s12185-017-2195-3. Epub 2017 Feb 9. Int J Hematol. 2017. PMID: 28185204
-
Optimization of In Vitro Expansion and Activation of Human Natural Killer Cells against Breast Cancer Cell Line.Avicenna J Med Biotechnol. 2020 Jan-Mar;12(1):17-23. Avicenna J Med Biotechnol. 2020. PMID: 32153734 Free PMC article.
-
Biosensors for Detecting Lymphocytes and Immunoglobulins.Biosensors (Basel). 2020 Oct 27;10(11):155. doi: 10.3390/bios10110155. Biosensors (Basel). 2020. PMID: 33121071 Free PMC article. Review.
-
Treatment outcomes and prognostic factors in adult patients with secondary hemophagocytic lymphohistiocytosis not associated with malignancy.Haematologica. 2019 Feb;104(2):269-276. doi: 10.3324/haematol.2018.198655. Epub 2018 Sep 13. Haematologica. 2019. PMID: 30213834 Free PMC article.
References
-
- Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends in Immunology. 2001;22(11):633–640. - PubMed
-
- Valiathan R, Lewis JE, Melillo AB, Leonard S, Ali KH, Asthana D. Evaluation of a flow cytometry-based assay for natural killer cell activity in clinical settings. Scandinavian Journal of Immunology. 2012;75(4):455–462. - PubMed
-
- Lee S-H, Miyagi T, Biron CA. Keeping NK cells in highly regulated antiviral warfare. Trends in Immunology. 2007;28(6):252–259. - PubMed
-
- Moretta L, Ciccone E, Poggi A, Mingari MC, Moretta A. Origin and functions of human natural killer cells. International Journal of Clinical & Laboratory Research. 1994;24(4):181–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

