Vesicular glutamate transporter 2 is required for the respiratory and parasympathetic activation produced by optogenetic stimulation of catecholaminergic neurons in the rostral ventrolateral medulla of mice in vivo
- PMID: 24236954
- PMCID: PMC4790426
- DOI: 10.1111/ejn.12421
Vesicular glutamate transporter 2 is required for the respiratory and parasympathetic activation produced by optogenetic stimulation of catecholaminergic neurons in the rostral ventrolateral medulla of mice in vivo
Abstract
Catecholaminergic neurons of the rostral ventrolateral medulla (RVLM-CA neurons; C1 neurons) contribute to the sympathetic, parasympathetic and neuroendocrine responses elicited by physical stressors such as hypotension, hypoxia, hypoglycemia, and infection. Most RVLM-CA neurons express vesicular glutamate transporter (VGLUT)2, and may use glutamate as a ionotropic transmitter, but the importance of this mode of transmission in vivo is uncertain. To address this question, we genetically deleted VGLUT2 from dopamine-β-hydroxylase-expressing neurons in mice [DβH(Cre/0) ;VGLUT2(flox/flox) mice (cKO mice)]. We compared the in vivo effects of selectively stimulating RVLM-CA neurons in cKO vs. control mice (DβH(Cre/0) ), using channelrhodopsin-2 (ChR2-mCherry) optogenetics. ChR2-mCherry was expressed by similar numbers of rostral ventrolateral medulla (RVLM) neurons in each strain (~400 neurons), with identical selectivity for catecholaminergic neurons (90-99% colocalisation with tyrosine hydroxylase). RVLM-CA neurons had similar morphology and axonal projections in DβH(Cre/0) and cKO mice. Under urethane anesthesia, photostimulation produced a similar pattern of activation of presumptive ChR2-positive RVLM-CA neurons in DβH(Cre/0) and cKO mice. Photostimulation in conscious mice produced frequency-dependent respiratory activation in DβH(Cre/0) mice but no effect in cKO mice. Similarly, photostimulation under urethane anesthesia strongly activated efferent vagal nerve activity in DβH(Cre/0) mice only. Vagal responses were unaffected by α1 -adrenoreceptor blockade. In conclusion, two responses evoked by RVLM-CA neuron stimulation in vivo require the expression of VGLUT2 by these neurons, suggesting that the acute autonomic responses driven by RVLM-CA neurons are mediated by glutamate.
Keywords: C1 neuron; channelrhodopsin 2; conditional knockout; gene disruption in mice; glutamate.
© 2013 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Figures
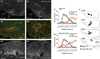
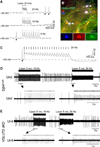
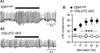
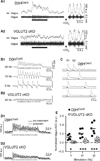
Comment in
-
C1 Neurons in the RVLM: are they catecholaminergic in name only? (Commentary on Abbott et al.).Eur J Neurosci. 2014 Jan;39(1):97. doi: 10.1111/ejn.12454. Eur J Neurosci. 2014. PMID: 24387592 No abstract available.
References
-
- Bellocchio EE, Reimer RJ, Fremeau RT, Jr, Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

