Signaling pathways controlling skeletal muscle mass
- PMID: 24237131
- PMCID: PMC3913083
- DOI: 10.3109/10409238.2013.857291
Signaling pathways controlling skeletal muscle mass
Abstract
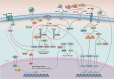
The molecular mechanisms underlying skeletal muscle maintenance involve interplay between multiple signaling pathways. Under normal physiological conditions, a network of interconnected signals serves to control and coordinate hypertrophic and atrophic messages, culminating in a delicate balance between muscle protein synthesis and proteolysis. Loss of skeletal muscle mass, termed "atrophy", is a diagnostic feature of cachexia seen in settings of cancer, heart disease, chronic obstructive pulmonary disease, kidney disease, and burns. Cachexia increases the likelihood of death from these already serious diseases. Recent studies have further defined the pathways leading to gain and loss of skeletal muscle as well as the signaling events that induce differentiation and post-injury regeneration, which are also essential for the maintenance of skeletal muscle mass. In this review, we summarize and discuss the relevant recent literature demonstrating these previously undiscovered mediators governing anabolism and catabolism of skeletal muscle.
Figures
References
-
- Alessi DR, Caudwell FB, et al. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996;399:333–8. - PubMed
-
- Blaauw B, Canato M, Agatea L, et al. Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J. 2009;23:3896–905. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
