Direct β-functionalization of cyclic ketones with aryl ketones via the merger of photoredox and organocatalysis
- PMID: 24237366
- PMCID: PMC3934322
- DOI: 10.1021/ja410478a
Direct β-functionalization of cyclic ketones with aryl ketones via the merger of photoredox and organocatalysis
Abstract
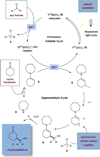
The direct β-coupling of cyclic ketones with aryl ketones has been achieved via the synergistic combination of photoredox catalysis and organocatalysis. Diaryl oxymethyl or aryl-alkyl oxymethyl radicals, transiently generated via single-electron reduction of ketone precursors, readily merge with β-enaminyl radical species, generated by photon-induced enamine oxidation, to produce γ-hydroxyketone adducts. Experimental evidence indicates that two discrete reaction pathways can be operable in this process depending upon the nature of the ketyl radical precursor and the photocatalyst.
Figures
References
-
- Carey FA, Sundberg RJ. Advanced Organic Chemistry: Part B: Reactions and Synthesis. New York: Springer; 2001.
-
- Mukherjee S, Yang JW, Hoffmann S, List B. Chem. Rev. 2007;107:5471. - PubMed
- Evans DA, Helmchen G, Rüping M. Asymmetric Synthesis–The Essentials. Wiley-VCH: Weinheim; 2006.
-
-
For direct β-functionalizations of amides, esters, or oximes, see: Zaitsev VG, Shabashov D, Daugulis O. J. Am. Chem. Soc. 2005;127:13154. Wasa M, Engle KM, Yu J-Q. J. Am. Chem. Soc. 2009;131:9886. Stowers KJ, Kubota A, Sanford MS. Chem. Sci. 2012;3:3192. Renaudat A, Jean-Gérard L, Jazzar R, Kefalidis CE, Clot E, Baudoin O. Angew. Chem. Int. Ed. 2010;49:7261.
-
-
- Yamamoto H. Lewis Acids in Organic Synthesis. New York: Wiley-VCH; 2000.
- Crabtree RH. The Organometallic Chemistry of the Transition Metals. Hoboken: Wiley-Interscience; 2005.
- Noyori R. Asymmetric Catalysis in Organic Synthesis. New York: Wiley-Interscience; 1994.
- Ojima I. Catalytic Asymmetric Synthesis. New York: Wiley-VCH; 2000.
- Lelais G, MacMillan DWC. Aldrichim. Acta. 2006;39:79.
- Taylor MS, Jacobsen EN. Angew. Chem. Int. Ed. 2006;45:1520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources