Humoral and cellular responses to a non-adjuvanted monovalent H1N1 pandemic influenza vaccine in hospital employees
- PMID: 24238117
- PMCID: PMC3835617
- DOI: 10.1186/1471-2334-13-544
Humoral and cellular responses to a non-adjuvanted monovalent H1N1 pandemic influenza vaccine in hospital employees
Abstract
Background: The efficacy of the H1N1 influenza vaccine relies on the induction of both humoral and cellular responses. This study evaluated the humoral and cellular responses to a monovalent non-adjuvanted pandemic influenza A/H1N1 vaccine in occupationally exposed subjects who were previously vaccinated with a seasonal vaccine.
Methods: Sixty healthy workers from a respiratory disease hospital were recruited. Sera and peripheral blood mononuclear cells (PBMCs) were obtained prior to and 1 month after vaccination with a non-adjuvanted monovalent 2009 H1N1 vaccine (Influenza A (H1N1) 2009 Monovalent Vaccine Panenza, Sanofi Pasteur). Antibody titers against the pandemic A/H1N1 influenza virus were measured via hemagglutination inhibition (HI) and microneutralization assays. Antibodies against the seasonal HA1 were assessed by ELISA. The frequency of IFN-γ-producing cells as well as CD4+ and CD8+ T cell proliferation specific to the pandemic virus A/H1N peptides, seasonal H1N1 peptides and seasonal H3N2 peptides were assessed using ELISPOT and flow cytometry.
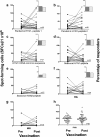
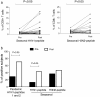
Results: At baseline, 6.7% of the subjects had seroprotective antibody titers. The seroconversion rate was 48.3%, and the seroprotection rate was 66.7%. The geometric mean titers (GMTs) were significantly increased (from 6.8 to 64.9, p < 0.05). Forty-nine percent of the subjects had basal levels of specific IFN-γ-producing T cells to the pandemic A/H1N1 peptides that were unchanged post-vaccination. CD4+ T cell proliferation in response to specific pandemic A/H1N1 virus peptides was also unchanged; in contrast, the antigen-specific proliferation of CD8+ T cells significantly increased post-vaccination.
Conclusion: Our results indicate that a cellular immune response that is cross-reactive to pandemic influenza antigens may be present in populations exposed to the circulating seasonal influenza virus prior to pandemic or seasonal vaccination. Additionally, we found that the pandemic vaccine induced a significant increase in CD8+ T cell proliferation.
Figures


References
-
- Virus strains recommended by the World Health Organization for the development of the influenza A (H1N1) vaccines. http://www.who.int/csr/resources/publications/swineflu/2009_05_27_X179Aa....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

