Engineered T cells for cancer treatment
- PMID: 24239105
- PMCID: PMC4013208
- DOI: 10.1016/j.jcyt.2013.10.002
Engineered T cells for cancer treatment
Abstract
Adoptively transferred T cells have the capacity to traffic to distant tumor sites, infiltrate fibrotic tissue and kill antigen-expressing tumor cells. Various groups have investigated different genetic engineering strategies designed to enhance tumor specificity, increase T cell potency, improve proliferation, persistence or migratory capacity and increase safety. This review focuses on recent developments in T cell engineering, discusses the clinical application of these engineered cell products and outlines future prospects for this therapeutic modality.
Keywords: CAR T cells; cancer treatment; genetic modification of T cells; immunotherapy.
Copyright © 2014 International Society for Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
UA has no COI to report, MKB is a scientific advisory board member of Jennerex and of Bluebird Bio. Present research in Center for Cell and Gene Therapy (of which he is director) receives support from CellMedica and the centre has a Research Collaboration with Celgene and Bluebird Bio. JFV is a scientific adviser for Wilson Wolf Manufacturing. AML, JFV and MKB have patent applications in the specialty of T cell and gene-modified T-cell therapy for cancer. All authors have disclosed any financial or personal relationship with organizations that could potentially be perceived as influencing the described research and all authors have read the journal’s policy on disclosure of potential conflicts of interest.
Figures

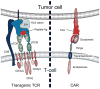
Similar articles
-
CAR T-cell Therapy: A New Era in Cancer Immunotherapy.Curr Pharm Biotechnol. 2018;19(1):5-18. doi: 10.2174/1389201019666180418095526. Curr Pharm Biotechnol. 2018. PMID: 29667553 Review.
-
A Multidrug-resistant Engineered CAR T Cell for Allogeneic Combination Immunotherapy.Mol Ther. 2015 Sep;23(9):1507-18. doi: 10.1038/mt.2015.104. Epub 2015 Jun 10. Mol Ther. 2015. PMID: 26061646 Free PMC article.
-
CARTs for Solid Tumors: Feasible or Infeasible?Oncol Res Treat. 2017;40(9):540-546. doi: 10.1159/000477095. Epub 2017 Aug 17. Oncol Res Treat. 2017. PMID: 28813706 Review.
-
Engineering T cells for adoptive therapy: outsmarting the tumor.Curr Opin Immunol. 2018 Apr;51:133-139. doi: 10.1016/j.coi.2018.03.014. Epub 2018 Mar 24. Curr Opin Immunol. 2018. PMID: 29579622 Review.
-
Programming CAR-T cells to kill cancer.Nat Biomed Eng. 2018 Jun;2(6):377-391. doi: 10.1038/s41551-018-0235-9. Epub 2018 Jun 11. Nat Biomed Eng. 2018. PMID: 31011197 Review.
Cited by
-
Electroporation of mRNA as a Universal Technology Platform to Transfect a Variety of Primary Cells with Antigens and Functional Proteins.Methods Mol Biol. 2024;2786:219-235. doi: 10.1007/978-1-0716-3770-8_10. Methods Mol Biol. 2024. PMID: 38814397
-
In vitro machine learning-based CAR T immunological synapse quality measurements correlate with patient clinical outcomes.PLoS Comput Biol. 2022 Mar 18;18(3):e1009883. doi: 10.1371/journal.pcbi.1009883. eCollection 2022 Mar. PLoS Comput Biol. 2022. PMID: 35303007 Free PMC article. Clinical Trial.
-
Analysis and Augmentation of the Immunologic Bystander Effects of CAR T Cell Therapy in a Syngeneic Mouse Cancer Model.Mol Ther Oncolytics. 2020 Jul 15;18:360-371. doi: 10.1016/j.omto.2020.07.005. eCollection 2020 Sep 25. Mol Ther Oncolytics. 2020. PMID: 32802940 Free PMC article.
-
Advancing Immune and Cell-Based Therapies Through Imaging.Mol Imaging Biol. 2017 Jun;19(3):379-384. doi: 10.1007/s11307-017-1069-7. Mol Imaging Biol. 2017. PMID: 28271366 Free PMC article. Review.
-
Towards a commercial process for the manufacture of genetically modified T cells for therapy.Cancer Gene Ther. 2015 Mar;22(2):72-8. doi: 10.1038/cgt.2014.78. Epub 2015 Jan 23. Cancer Gene Ther. 2015. PMID: 25613483 Free PMC article. Review.
References
-
- Dudley ME, Wunderlich J, Nishimura MI, Yu D, Yang JC, Topalian SL, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

