Suprazero cooling rate, rather than freezing rate, determines post thaw quality of rhesus macaque sperm
- PMID: 24239181
- PMCID: PMC3893114
- DOI: 10.1016/j.theriogenology.2013.10.008
Suprazero cooling rate, rather than freezing rate, determines post thaw quality of rhesus macaque sperm
Abstract
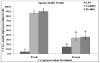
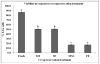
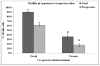
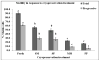
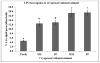
Sperm become most sensitive to cold shock when cooled from 37 °C to 5 °C at rates that are too fast or too slow; cold shock increases the susceptibility to oxidative damage owing to its influence on reactive oxygen species (ROS) production, which are significant stress factors generated during cooling and low temperature storage. In addition, ROS may be a main cause of decreased motility and fertility upon warming. They have been shown to change cellular function through the disruption of the sperm plasma membrane and through damage to proteins and DNA. The objective of this study was to determine which cryopreservation rates result in the lowest degree of oxidative damage and greatest sperm quality. In the rhesus model, it has not been determined whether suprazero cooling or subzero freezing rates causes a significant amount of ROS damage to sperm. Semen samples were collected from male rhesus macaques, washed, and resuspended in TEST-yolk cryopreservation buffer to 100 × 10(6) sperm/mL. Sperm were frozen in 0.5-mL straws at four different combinations of suprazero and subzero rates. Three different suprazero rates were used between 22 °C and 0 °C: 0.5 °C/min (slow), 45 °C/min (medium), and 93 °C/min (fast). These suprazero rates were used in combination with two different subzero rates for temperatures 0 °C to -110 °C: 42 °C/min (medium) and 87 °C/min (fast). The different freezing groups were as follows: slow-med (SM), slow-fast (SF), med-med (MM), and fast-fast (FF). Flow cytometry was used to detect lipid peroxidation (LPO), a result of ROS generation. Motility was evaluated using a computer assisted sperm motion analyzer. The MM and FF treated sperm had less viable (P < 0.0001) and motile sperm (P < 0.001) than the SM, SF, or fresh sperm. Sperm exposed to MM and FF treatments demonstrated significantly higher oxidative damage than SM, SF, or fresh sperm (P < 0.05). The SM- and SF-treated sperm showed decreased motility, membrane integrity, and LPO compared with fresh semen (P < 0.001). Slow cooling from room temperature promotes higher membrane integrity and motility post thaw, compared with medium or fast cooling rates. Cells exposed to similar cooling rates with differing freezing rates were not different in motility and membrane integrity, whereas comparison of cells exposed to differing cooling rates with similar freezing rates indicated significant differences in motility, membrane integrity, and LPO. These data suggest that sperm quality seems to be more sensitive to the cooling, rather than freezing rate and highlight the role of the suprazero cooling rate in post thaw sperm quality.
Keywords: Cryopreservation; Lipid peroxidation; Rhesus; Sperm.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Quantification of damage at different stages of cryopreservation of endangered North American bison (Bison bison) semen and the effects of extender and freeze rate on post-thaw sperm quality.Anim Reprod Sci. 2011 Dec;129(3-4):171-9. doi: 10.1016/j.anireprosci.2011.12.002. Epub 2011 Dec 9. Anim Reprod Sci. 2011. PMID: 22240453
-
Cryopreservation of bull semen shipped overnight and its effect on post-thaw sperm motility, plasma membrane integrity, mitochondrial membrane potential and normal acrosomes.Anim Reprod Sci. 2011 Jun;126(1-2):23-31. doi: 10.1016/j.anireprosci.2011.04.018. Epub 2011 May 4. Anim Reprod Sci. 2011. PMID: 21621352
-
Effect of cooling rate on sperm quality of cryopreserved Andalusian donkey spermatozoa.Anim Reprod Sci. 2018 Jun;193:201-208. doi: 10.1016/j.anireprosci.2018.04.069. Epub 2018 Apr 20. Anim Reprod Sci. 2018. PMID: 29699919
-
Cryopreservation of domestic animal sperm cells.Cell Tissue Bank. 2009 Feb;10(1):49-62. doi: 10.1007/s10561-008-9081-4. Epub 2008 Jun 12. Cell Tissue Bank. 2009. PMID: 18548333 Review.
-
Cryopreservation Techniques for Ram Sperm.Vet Med Int. 2022 Apr 30;2022:7378379. doi: 10.1155/2022/7378379. eCollection 2022. Vet Med Int. 2022. PMID: 35535035 Free PMC article. Review.
Cited by
-
Semen collection by urethral catheterization and electro-ejaculation with different voltages, and the effect of holding temperature and cooling rate before cryopreservation on semen quality in the Japanese macaque (Macaca fuscata).J Vet Med Sci. 2022 Mar 15;84(3):429-438. doi: 10.1292/jvms.21-0590. Epub 2022 Jan 24. J Vet Med Sci. 2022. PMID: 35067494 Free PMC article.
-
Best practices for cryopreserving sperm in Nonhuman Primates: a systematic review and meta-analysis.Sci Rep. 2025 Jan 31;15(1):3947. doi: 10.1038/s41598-025-88226-7. Sci Rep. 2025. PMID: 39890990 Free PMC article.
-
Cryopreservation and Preparation of Thawed Spermatozoa from Rhesus Macaques (Macaca mulatta) for In Vitro Fertilization.J Am Assoc Lab Anim Sci. 2021 Jul 1;60(4):396-406. doi: 10.30802/AALAS-JAALAS-20-000028. Epub 2021 May 23. J Am Assoc Lab Anim Sci. 2021. PMID: 34024310 Free PMC article.
-
The optimal combination of cooling and equilibration durations, along with the addition of melatonin, gamma-oryzanol, and canthaxanthin, for improving swamp buffalo semen cryopreservation quality.Vet World. 2024 Dec;17(12):2950-2956. doi: 10.14202/vetworld.2024.2950-2956. Epub 2024 Dec 26. Vet World. 2024. PMID: 39897372 Free PMC article.
-
Cryopreservation of Mauritian Cynomolgus Macaque (Macaca fascicularis) Sperm in Chemically Defined Medium.J Am Assoc Lab Anim Sci. 2020 Nov 1;59(6):681-686. doi: 10.30802/AALAS-JAALAS-20-000059. Epub 2020 Sep 2. J Am Assoc Lab Anim Sci. 2020. PMID: 32878681 Free PMC article.
References
-
- Bucak MN, Atessahin A, Yuce A. Effect of anti-oxidants and oxidative stress parameters on ram semen after the freeze-thawing process. Small Rum Res. 2008;77:89.
-
- Drobnis EZ, Crowe LM, Berger T, Anchordoguy TJ, Overstreet JW, Crowe JH. Cold shock damage is due to lipid phase transitions in cell membranes: a demonstration using sperm as a model. J Exp Zool. 1993;265:432–7. - PubMed
-
- Ricker JV, Linfor JJ, Delfino WJ, Kysar P, Scholtz EL, Tablin F, et al. Equine sperm membrane phase behavior: the effects of lipid-based cryoprotectants. Biol Reprod. 2006;74:359–65. - PubMed
-
- Baumber J, Ball BA, Linfor JJ. Assessment of the cryopreservation of equine spermatozoa in the presence of enzyme scavengers and antioxidants. Am J Vet Res. 2005;66:772–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

