Fluorouracil, epirubicin, and cyclophosphamide (FEC-75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC-75 plus trastuzumab as neoadjuvant treatment for patients with HER2-positive breast cancer (Z1041): a randomised, controlled, phase 3 trial
- PMID: 24239210
- PMCID: PMC4176878
- DOI: 10.1016/S1470-2045(13)70502-3
Fluorouracil, epirubicin, and cyclophosphamide (FEC-75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC-75 plus trastuzumab as neoadjuvant treatment for patients with HER2-positive breast cancer (Z1041): a randomised, controlled, phase 3 trial
Abstract
Background: Neoadjuvant chemotherapy with trastuzumab for patients with HER2-positive breast cancer can produce a pathological complete response in the breast in 30-65% of patients. We investigated the effect of the timing of trastuzumab administration with anthracycline and taxane neoadjuvant chemotherapy.
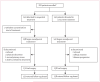
Methods: This randomised trial was done at 36 centres in the USA and Puerto Rico. Women with operable HER2-positive invasive breast cancer were randomly assigned (1:1) with a biased coin minimisation algorithm, stratified for age, tumour size, and hormone receptor status. Neither patients nor investigators (except for a cardiac safety review panel) were masked to treatment assignment. Patients randomly assigned to sequential treatment received fluorouracil 500 mg/m(2), epirubicin 75 mg/m(2), and cyclophosphamide 500 mg/m(2) (FEC-75) on day 1 of a 21-day cycle for four cycles followed by paclitaxel 80 mg/m(2) and trastuzumab 2 mg/kg (after a 4 mg/kg loading dose) once per week for 12 weeks, while those randomly assigned to the concurrent treatment group received paclitaxel and trastuzumab once per week for 12 weeks followed by four cycles of FEC-75 (on day 1 of each 21-day cycle) and once-weekly trastuzumab, in the same doses as the sequential group. Surgery, including evaluation of the axilla, was done within 6 weeks of completion of neoadjuvant treatment. The primary outcome was the percentage of patients who had a pathological complete response in the intention-to-treat population. The study is registered with ClinicalTrials.gov, number NCT00513292.
Findings: From Sept 15, 2007, to Dec 15, 2011, 282 women were enrolled (140 in the sequential group, 142 in the concurrent group). Two patients in the sequential group withdrew consent before starting treatment. 78 of 138 (56·5%, 95% CI 47·8-64·9) patients who received sequential treatment had a pathological complete response in the breast versus 77 of 142 (54·2%, 95% CI 45·7-62·6) who received concurrent treatment (difference 2·3%, 95% CI -9·3 to 13·9). No treatment-related deaths occurred. The most common severe toxic effects were neutropenia (35 [25·3%] of 138 patients in the sequential group vs 45 [31·7%] of 142 patients in the concurrent group) and fatigue (six [4·3%] vs 12 [8·5%]). Left ventricular ejection fraction dropped below the institutional lower limit of normal at week 12 in one (0·8%) of 130 patients who received sequential treatment and four (2·9%) of 137 patients who received concurrent treatment; by week 24, it had dropped below this limit in nine (7·1%) of 126 patients and in six (4·6%) of 130 patients, respectively.
Interpretation: Concurrent administration of trastuzumab with anthracyclines offers no additional benefit and is not warranted.
Funding: US National Cancer Institute.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Treatment of HER2-positive breast cancer: looking backwards briefly.Lancet Oncol. 2013 Dec;14(13):1250-1. doi: 10.1016/S1470-2045(13)70536-9. Epub 2013 Nov 13. Lancet Oncol. 2013. PMID: 24239207 No abstract available.
-
[No addition benefit from coadministration of trastuzumab and anthracyclines].Strahlenther Onkol. 2014 Apr;190(4):428-9. doi: 10.1007/s00066-014-0558-8. Strahlenther Onkol. 2014. PMID: 24638245 German. No abstract available.
References
-
- Fisher B, Brown A, Mamounas E, et al. Effect of preopera tive chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–93. - PubMed
-
- Buzdar AU. Preoperative chemotherapy treatment of breast cancer—a review. Cancer. 2007;110:2394–407. - PubMed
-
- Gralow JR, Burstein HJ, Wood W, et al. Preoperative ther apy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol. 2008;26:814–19. - PubMed
-
- Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366:2438–41. - PubMed
-
- von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–804. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

