Multiple conformational selection and induced fit events take place in allosteric propagation
- PMID: 24239303
- PMCID: PMC6361548
- DOI: 10.1016/j.bpc.2013.10.002
Multiple conformational selection and induced fit events take place in allosteric propagation
Abstract
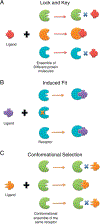
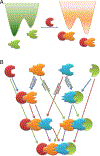
The fact that we observe a single conformational selection event during binding does not necessarily mean that only a single conformational selection event takes place, even though this is the common assumption. Here we suggest that conformational selection takes place not once in a given binding/allosteric event, but at every step along the allosteric pathway. This view generalizes conformational selection and makes it applicable also to other allosteric events, such as post-translational modifications (PTMs) and photon absorption. Similar to binding, at each step along a propagation pathway, conformational selection is coupled with induced fit which optimizes the interactions. Thus, as in binding, the allosteric effects induced by PTMs and light relate not only to population shift; but to conformational selection as well. Conformational selection and population shift take place conjointly.
Keywords: Allosteric; Allostery; Binding; Conformational selection; Induced fit; Molecular recognition.
© 2013 Elsevier B.V. All rights reserved.
Figures
References
-
- Fischer E, Einfluss der configuration auf die wirkung der enzyme, Ber. Dtsch. Chem. Ges. 27 (1894) 2984–2993.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

