Alternate splicing of dysferlin C2A confers Ca²⁺-dependent and Ca²⁺-independent binding for membrane repair
- PMID: 24239457
- PMCID: PMC5993433
- DOI: 10.1016/j.str.2013.10.001
Alternate splicing of dysferlin C2A confers Ca²⁺-dependent and Ca²⁺-independent binding for membrane repair
Abstract
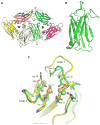
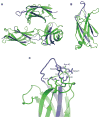
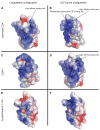
Dysferlin plays a critical role in the Ca²⁺-dependent repair of microlesions that occur in the muscle sarcolemma. Of the seven C2 domains in dysferlin, only C2A is reported to bind both Ca²⁺ and phospholipid, thus acting as a key sensor in membrane repair. Dysferlin C2A exists as two isoforms, the "canonical" C2A and C2A variant 1 (C2Av1). Interestingly, these isoforms have markedly different responses to Ca²⁺ and phospholipid. Structural and thermodynamic analyses are consistent with the canonical C2A domain as a Ca²⁺-dependent, phospholipid-binding domain, whereas C2Av1 would likely be Ca²⁺-independent under physiological conditions. Additionally, both isoforms display remarkably low free energies of stability, indicative of a highly flexible structure. The inverted ligand preference and flexibility for both C2A isoforms suggest the capability for both constitutive and Ca²⁺-regulated effector interactions, an activity that would be essential in its role as a mediator of membrane repair.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures



References
-
- Ampong BN, Imamura M, Matsumiya T, Yoshida M, Takeda S. Intracellular localization of dysferlin and its association with the dihydropyridine receptor. Acta Myol. 2005;24:134–144. - PubMed
-
- Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

