Production of adeno-associated virus (AAV) serotypes by transient transfection of HEK293 cell suspension cultures for gene delivery
- PMID: 24239634
- PMCID: PMC7113661
- DOI: 10.1016/j.jviromet.2013.10.038
Production of adeno-associated virus (AAV) serotypes by transient transfection of HEK293 cell suspension cultures for gene delivery
Abstract
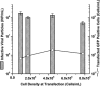
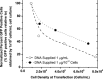
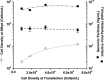
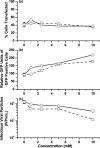
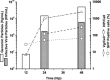
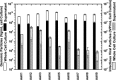
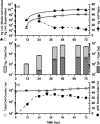
Adeno-associated virus (AAV) is being used successfully in gene therapy. Different serotypes of AAV target specific organs and tissues with high efficiency. There exists an increasing demand to manufacture various AAV serotypes in large quantities for pre-clinical and clinical trials. A generic and scalable method has been described in this study to efficiently produce AAV serotypes (AAV1-9) by transfection of a fully characterized cGMP HEK293SF cell line grown in suspension and serum-free medium. First, the production parameters were evaluated using AAV2 as a model serotype. Second, all nine AAV serotypes were produced successfully with yields of 10(13)Vg/L cell culture. Subsequently, AAV2 and AAV6 serotypes were produced in 3-L controlled bioreactors where productions yielded up to 10(13)Vg/L similar to the yields obtained in shake-flasks. For example, for AAV2 10(13)Vg/L cell culture (6.8×10(11)IVP/L) were measured between 48 and 64h post transfection (hpt). During this period, the average cell specific AAV2 yields of 6800Vg per cell and 460IVP per cell were obtained with a Vg to IVP ratio of less than 20. Successful operations in bioreactors demonstrated the potential for scale-up and industrialization of this generic process for manufacturing AAV serotypes efficiently.
Keywords: Bioreactor; Gene therapy; Large-scale transient transfection; Manufacturing; Process; Production.
Crown Copyright © 2013. Published by Elsevier B.V. All rights reserved.
Figures


References
-
- Ansorge S., Lanthier S., Transfiguracion J., Durocher Y., Henry O., Kamen A. Development of a scalable process for high-yield lentiviral vector production by transient transfection of HEK293 suspension cultures. J. Gene Med. 2009;11:868–876. - PubMed
-
- Asokan A., Conway J.C., Phillips J.L., Li C., Hegge J., Sinnott R., Yadav S., DiPrimio N., Nam H.J., Agbandje-McKenna M., McPhee S., Wolff J., Samulski R.J. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nat. Biotechnol. 2010;28:79–82. - PMC - PubMed
-
- Aucoin M.G., Perrier M., Kamen A.A. Critical assessment of current adeno-associated viral vector production and quantification methods. Biotechnol. Adv. 2008;26:73–88. - PubMed
-
- Backliwal G., Hildinger M., Kuettel I., Delegrange F., Hacker D.L., Wurm F.M. Valproic acid: a viable alternative to sodium butyrate for enhancing protein expression in mammalian cell cultures. Biotechnol. Bioeng. 2008;101:182–189. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

