Tumor growth rate is an early indicator of antitumor drug activity in phase I clinical trials
- PMID: 24240109
- PMCID: PMC3947306
- DOI: 10.1158/1078-0432.CCR-13-2098
Tumor growth rate is an early indicator of antitumor drug activity in phase I clinical trials
Abstract
Purpose: Response Evaluation Criteria in Solid Tumors (RECIST) evaluation does not take into account the pretreatment tumor kinetics and may provide incomplete information about experimental drug activity. Tumor growth rate (TGR) allows for a dynamic and quantitative assessment of the tumor kinetics. How TGR varies along the introduction of experimental therapeutics and is associated with outcome in phase I patients remains unknown.
Experimental design: Medical records from all patients (N = 253) prospectively treated in 20 phase I trials were analyzed. TGR was computed during the pretreatment period (reference) and the experimental period. Associations between TGR, standard prognostic scores [Royal Marsden Hospital (RMH) score], and outcome [progression-free survival (PFS) and overall survival (OS)] were computed (multivariate analysis).
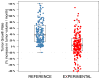
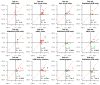
Results: We observed a reduction of TGR between the reference versus experimental periods (38% vs. 4.4%; P < 0.00001). Although most patients were classified as stable disease (65%) or progressive disease (25%) by RECIST at the first evaluation, 82% and 65% of them exhibited a decrease in TGR, respectively. In a multivariate analysis, only the decrease of TGR was associated with PFS (P = 0.004), whereas the RMH score was the only variable associated with OS (P = 0.0008). Only the investigated regimens delivered were associated with a decrease of TGR (P < 0.00001, multivariate analysis). Computing TGR profiles across different clinical trials reveals specific patterns of antitumor activity.
Conclusions: Exploring TGR in phase I patients is simple and provides clinically relevant information: (i) an early and subtle assessment of signs of antitumor activity; (ii) independent association with PFS; and (iii) it reveals drug-specific profiles, suggesting potential utility for guiding the further development of the investigational drugs.
Conflict of interest statement
Figures

Comment in
-
TGR analysis in phase I clinical trials--letter.Clin Cancer Res. 2014 May 1;20(9):2495-6. doi: 10.1158/1078-0432.CCR-13-3455. Clin Cancer Res. 2014. PMID: 24789035 No abstract available.
-
TGR analysis in phase I clinical trials--response.Clin Cancer Res. 2014 May 1;20(9):2497. doi: 10.1158/1078-0432.CCR-14-0366. Clin Cancer Res. 2014. PMID: 24789036 No abstract available.
References
-
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New Guidelines to Evaluate the Response to Treatment. 2000:92. - PubMed
-
- Eisenhauer E, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer Elsevier Ltd. 2009;45:228–47. - PubMed
-
- Maitland ML, Bies RR, Barrett JS. A time to keep and a time to cast away categories of tumor response. J Clin Oncol. 2011;29:3109–11. - PubMed
-
- Crabb SJ, Patsios D, Sauerbrei E, Ellis PM, Arnold A, Goss G, et al. Tumor cavitation: impact on objective response evaluation in trials of angiogenesis inhibitors in non-small-cell lung cancer. J Clin Oncol. 2009 - PubMed
-
- Benjamin RS, Choi H, Macapinlac Ha, Burgess Ma, Patel SR, Chen LL, et al. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

