Immunogenicity and safety of an inactivated 2012/2013 trivalent influenza vaccine produced in mammalian cell culture (Optaflu®): an open label, uncontrolled study
- PMID: 24240428
- PMCID: PMC4185887
- DOI: 10.4161/hv.27140
Immunogenicity and safety of an inactivated 2012/2013 trivalent influenza vaccine produced in mammalian cell culture (Optaflu®): an open label, uncontrolled study
Abstract
Background: The present study aimed to evaluate immunogenicity and safety of the 2012/2013 seasonal influenza vaccine (Optaflu(®)) after the World Health Organization recommended two new strains for the composition.
Results: Twenty-one days post-vaccination geometric mean titers (GMTs) against A(H1N1), A(H3N2) and the B strain were 528, 935, and 201 for adults and 272, 681, and 101 for elderly subjects, respectively. The proportion of subjects with a HI titer of ≥ 40 against the three strains A(H1N1), A(H3N2) and B was 98%, 100%, and 98% in adults and 100%, 100%, and 85% in elderly subjects, respectively. Optaflu(®) met the CHMP criteria of the Committee for Medicinal Products for Human Use (CPMP/BWP/214/96). Pre-vaccination titers indicated seroprotection against the A(H1N1), the A(H3N2) and the B strain in 56%, 86%, and 54% of the adults and in 61%, 85%, and 40% of the elderly with highest titers against the A(H3N2) strain. In the safety analysis injection site pain (37%) and myalgia (31%) were the most common local and systemic reactions. No serious adverse events were recorded.
Conclusion: The 2012/2013 seasonal influenza vaccine Optaflu(®) showed good immunogenicity and an acceptable safety profile in both adults and elderly.
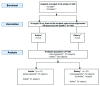
Methods: In this trial, 126 subjects (63 adults ≥18 to ≤60 y, 63 elderly ≥61 y) were vaccinated with a single dose Optaflu(®) containing each of the three virus strains recommended for the 2012/2013 season (A/California/7/2009(H1N1)-like strain, A/Victoria/361/2011(H3N2)-like strain, and B/Wisconsin/1/2010-like strain). Immunogenicity was assessed by hemagglutinin inhibition (HI) and single radial hemolysis (SRH) assays on day 22, the safety profile was investigated throughout the whole study period.
Keywords: H1N1; H3N2; Optaflu; influenza; pandemic; trivalent vaccine.
Figures
References
-
- World Health Organization. (2009) Influenza (seasonal) Available: http://www.who.int/mediacentre/factsheets/fs211/en/index.html Accessed: 06.12.2012
-
- Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, Bresee JS, Cox NS, Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57(RR-7):1–60. - PubMed
-
- Center for Disease Control and Prevention (CDC). (2012) Update: Influenza Activity — United States, 2011–12 Season and Composition of the 2012–13 Influenza Vaccine Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a4.htm Accessed: 06.12.2012. - PubMed
-
- Center for Disease Control and Prevention (CDC). (2012) Flu Symptoms and Severity Available: http://www.cdc.gov/flu/about/disease/symptoms.htm Accessed: 06.12.2012
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
