Fluoxetine epigenetically alters the CaMKIIα promoter in nucleus accumbens to regulate ΔFosB binding and antidepressant effects
- PMID: 24240473
- PMCID: PMC3957112
- DOI: 10.1038/npp.2013.319
Fluoxetine epigenetically alters the CaMKIIα promoter in nucleus accumbens to regulate ΔFosB binding and antidepressant effects
Abstract
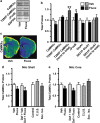
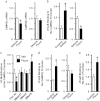
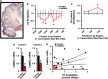
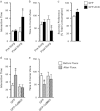
Chronic social defeat stress in mice produces a susceptible phenotype characterized by several behavioral abnormalities consistent with human depression that are reversed by chronic but not acute exposure to antidepressant medications. Recent work in addiction models demonstrates that the transcription factor ΔFosB and protein kinase calmodulin-dependent protein kinase II (CaMKII) are co-regulated in nucleus accumbens (NAc), a brain reward region implicated in both addiction and depression models including social defeat. Previous work has also demonstrated that ΔFosB is induced in NAc after chronic social defeat stress or after chronic antidepressant treatment, wherein it mediates a pro-resilience or antidepressant-like phenotype. Here, using chromatin immunoprecipitation assays, we found that ΔFosB binds the CaMKIIα gene promoter in NAc and that this binding increases after mice are exposed to chronic social defeat stress. Paradoxically, chronic exposure to the antidepressant fluoxetine reduces binding of ΔFosB to the CaMKIIα promoter and reduces CaMKII expression in NAc, despite the fact that ΔFosB is induced under these conditions. These data suggest a novel epigenetic mechanism of antidepressant action, whereby fluoxetine induces some chromatin change at the CaMKIIα promoter, which blocks the ΔFosB binding. Indeed, chronic fluoxetine reduces acetylation and increases lysine-9 dimethylation of histone H3 at the CaMKIIα promoter in NAc, effects also seen in depressed humans exposed to antidepressants. Overexpression of CaMKII in NAc blocks fluoxetine's antidepressant effects in the chronic social defeat paradigm, whereas inhibition of CaMKII activity in NAc mimics fluoxetine exposure. These findings suggest that epigenetic suppression of CaMKIIα expression in NAc is behaviorally relevant and offer a novel pathway for possible therapeutic intervention in depression and related syndromes.
Figures
References
-
- Andrade AL, Abrahao KP, Goeldner FO, Souza-Formigoni ML. Administration of the 5-HT2C receptor antagonist SB-242084 into the nucleus accumbens blocks the expression of ethanol-induced behavioral sensitization in Albino Swiss mice. Neuroscience. 2011;189:178–186. - PubMed
-
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

