Mechanism and synergism in epithelial fluid and electrolyte secretion
- PMID: 24240699
- PMCID: PMC4024104
- DOI: 10.1007/s00424-013-1390-1
Mechanism and synergism in epithelial fluid and electrolyte secretion
Abstract
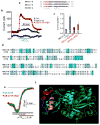
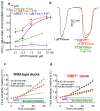
A central function of epithelia is the control of the volume and electrolyte composition of bodily fluids through vectorial transport of electrolytes and the obligatory H2O. In exocrine glands, fluid and electrolyte secretion is carried out by both acinar and duct cells, with the portion of fluid secreted by each cell type varying among glands. All acinar cells secrete isotonic, plasma-like fluid, while the duct determines the final electrolyte composition of the fluid by absorbing most of the Cl(-) and secreting HCO3 (-). The key transporters mediating acinar fluid and electrolyte secretion are the basolateral Na(+)/K(+) /2Cl(-) cotransporter, the luminal Ca(2+)-activated Cl(-) channel ANO1 and basolateral and luminal Ca(2+)-activated K(+) channels. Ductal fluid and HCO3 (-) secretion are mediated by the basolateral membrane Na(+)-HCO3 (-) cotransporter NBCe1-B and the luminal membrane Cl(-)/HCO3 (-) exchanger slc26a6 and the Cl(-) channel CFTR. The function of the transporters is regulated by multiple inputs, which in the duct include major regulation by the WNK/SPAK pathway that inhibit secretion and the IRBIT/PP1 pathway that antagonize the effects of the WNK/SPAK pathway to both stimulate and coordinate the secretion. The function of these regulatory pathways in secretory glands acinar cells is yet to be examined. An important concept in biology is synergism among signaling pathways to generate the final physiological response that ensures regulation with high fidelity and guards against cell toxicity. While synergism is observed in all epithelial functions, the molecular mechanism mediating the synergism is not known. Recent work reveals a central role for IRBIT as a third messenger that integrates and synergizes the function of the Ca(2+) and cAMP signaling pathways in activation of epithelial fluid and electrolyte secretion. These concepts are discussed in this review using secretion by the pancreatic and salivary gland ducts as model systems.
Figures



References
-
- Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem. 1998;273:17689–95. - PubMed
-
- Alexander RT, Grinstein S. Na+/H+ exchangers and the regulation of volume. Acta Physiol (Oxf) 2006;187:159–67. - PubMed
-
- Anderova M, Duchene AD, Barbara JG, Takeda K. Vasoactive intestinal peptide potentiates and directly stimulates catecholamine secretion from rat adrenal chromaffin cells. Brain Res. 1998;809:97–106. - PubMed
-
- Ando H, Mizutani A, Kiefer H, Tsuzurugi D, Michikawa T, Mikoshiba K. IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor. Mol Cell. 2006;22:795–806. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

